- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Sandoz Study Supports Expanded Use of Biosimilar Pegfilgrastim
Expanding access to biosimilar pegfilgrastim (Ziextenzo) for patients at intermediate risk of febrile neutropenia and converting patients at high risk who are on the reference product (Neulasta) could save millions in healthcare costs, according to new study results.
A new study funded by Sandoz demonstrated that expanding access to biosimilar pegfilgrastim (Ziextenzo) to patients at intermediate risk of developing febrile neutropenia (FN) and converting patients at high risk who are already taking the reference product (Neulasta) could save millions of dollars in healthcare costs.
“Expanding biosimilar pegfilgrastim prophylaxis to patients at intermediate risk of FN would not only reduce total costs but also reduce healthcare resource utilization, including hospitalization and emergency department visits,” said Weijia Wang, MSc, an associate director at Sandoz and the study’s lead author.
Reference pegfilgrastim tends to be prescribed only for patients with cancer who are at a high risk of FN, rather than for those who are at intermediate and low risk, due to its high cost.
Where Patients Can See Savings
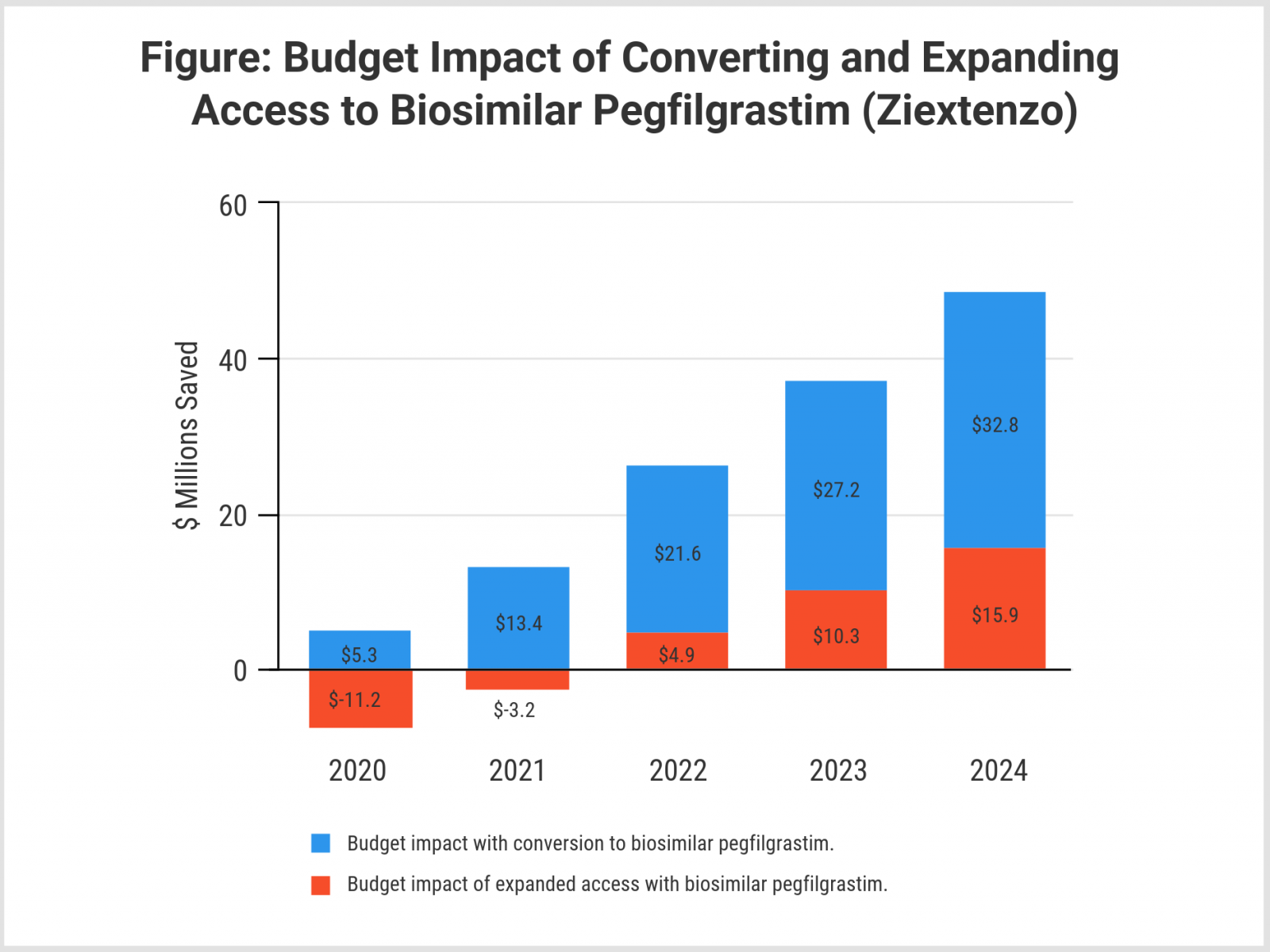
The investigators created a 5-year projection of savings from converting patients from reference to biosimilar pegfilgrastim and applied it to a hypothetical 1-million member health plan with a mixture of commercial payer and Medicare plan participants. Converting patients at high risk of FN to the biosimilar would have resulted in savings of $5.3 million in 2020, rising to $32.8 million by 2024 (Figure), they said.
A second 5-year projection evaluated potential savings from expanding access to biosimilar pegfilgrastim in patients with nonmyeloid cancer at intermediate risk of FN. Although drug costs would rise initially for this subgroup when given access to biosimilar pegfilgrastim, expanded access would begin to save money in hospital stays and emergency department and outpatient visits starting in year 3, according to the model. Estimates showed that this expanded access would generate $15.9 million in savings by 2024 (Figure).
On average, hospitalization can cost $27,155; emergency department visits with no hospitalization can cost $9727; and outpatient visits can cost $1664. Each FN episode can cost anywhere from $7100 to $34,756, Wang added.
“Contrary to the belief that expanding patient treatment translates to higher costs, we demonstrated that the reduction in healthcare resource utilization due to prophylaxis treatment far outweighs the increased treatment costs,” Wang said.
Background on Febrile Neutropenia
FN is the condition of having a low white blood cell count as a result of chemotherapy. It is one of the most serious and costly results of chemotherapy but can be prevented with the use of pegfilgrastim products.
“FN is a major dose-limiting toxicity of chemotherapy that can result in prolonged hospitalization, broad-spectrum antibiotic use, dose reductions, or treatment delays in subsequent chemotherapy cycles, and compromised clinical outcomes,” Wang said.
How Low-Risk Patients Measure Up
Wang also suggested that patients with low risk of FN might benefit from biosimilar pegfilgrastim, saying “We could consider conducting another research study on this population in the future.”
Current National Comprehensive Cancer Network hematopoietic growth factor guidelines do not recommend pegfilgrastim products for patients at low risk of developing FN. Patients who are at low risk have less than a 10% chance of developing FN.
A more thorough report on the Sandoz FN study is under development, Wang said. “We plan to develop a manuscript on this study and will publish it in a peer-reviewed journal.”
A poster on the study was presented during the Academy of Managed Care Pharmacy’s AMCP eLearning Days virtual meeting in April 2020.
Reference
Wang W, Li E, Campbell K, Ribes D. A budget impact analysis on the prophylactic use of biosimilar pegfilgrastim-bmez in non-myeloid cancer patients at risk of chemotherapy-induced febrile neutropenia and its expanded use to intermediate-risk patients. Presented at: AMCP eLearning Days; April 20-24, 2020; Alexandria, VA. Poster C9. https://plan.core-apps.com/amcp2020/abstract/a952fc2ad5de2fbd1164d14c6bd289bc.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
