- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Rising Costs Explain Why Canada Is Switching to Biosimilars
Following in the footsteps of British Columbia, other Canadian provinces are working to implement their own biosimilars initiatives that would switch patients from some of the most costly reference biologics to biosimilar counterparts.
A look at the numbers explains why Canadian provinces are introducing automatic biosimilar switching policies.
In Ontario, one of 4 provinces that are moving forward with forced switching, a total of $800,000 was spent on publicly funded biologic medications in 2018, up nearly 3-fold from $259.4 million in 2010, and the projection is for the total to reach $1 billion in 2021, according to a recent report.
However, in Ontario in the second quarter of 2019, biosimilar spending amounted to just 3.6% of the spending total (n = 4300 of 120,247 biologics users). Without a switching policy, and based on the rapid increase in biologics use, the proportion of biosimilar users is actually expected to drop to 3.4% of all biologics users by the second quarter of 2022.
“Initiatives should be explored that could increase the utilization of biosimilars, given the large potential for cost savings for the government,” authors of the report, the Ontario Drug Policy Research Network, concluded in the January 2020 review.
Initiative Pushbacks for Ontario
Not long after, in February, The Globe and Mail, a Canadian news publication, reported that Ontario had taken decisive steps to introduce a switching policy for biosimilars. If the province moves forward with this, it won’t be without having been tempted to maintain the status quo.
Janssen, the maker of reference infliximab (Remicade), provided the drug free of cost to thousands of patients in Canada over 4 years in what the Globe described as an attempt to discourage official policies favoring lower-cost competitors.
And patient advocates, not just in Ontario, have expressed consternation about forced switching for nonmedical reasons and the consequences for patients who may respond less favorably to biosimilar substitutes.
“Government-led initiatives have never shown us so clearly how invasive their roles can become in the determination of our healthcare treatments,” wrote Gail Attara, president and CEO of the Gastrointestinal Society and president of the Canadian Society of Intestinal Research.
“Forcing a patient to switch their medicine for nonmedical reasons takes health care management away from doctors and might put patients at risk. Most Canadian gastroenterologists and their association do not support a forced-switch policy. Research is absent on the long-term impacts such a policy can have on the safety of inflammatory bowel disease and other patients,” she wrote.
Yet more statistics may shed light on why these patient groups are having so much difficulty getting public officials to accept their arguments against nonmedical switching. Although in 2018 reference biologics accounted for just under 2% of prescribed drugs in Canada, they also accounted for nearly 30% of the total national drug cost, according to IQVIA. And private and public drug plans across Canada could save between $244 million to $1.33 billion, according to the Patented Medicines Prices Review Board.
British Columbia’s Biosimilars Initiative
Canadian authorities are moving ahead with biosimilar switching polices. British Columbia (BC), the first of the 4 Canadian provinces to have engaged with this process, has already published numbers showing that high proportions of patients have been switched to biosimilars.
In May of 2019, BC PharmaCare announced a Biosimilars Initiative that would expand the use of biosimilars and generate savings for the health care system by switching patients from 3 reference products to their biosimilar counterparts.
“The Biosimilars Initiative is a result of PharmaCare’s evidence-informed strategy to better optimize our public resources, get the best value for new treatments and services, and improve access to biologic medications for patients,” BC government officials said in a statement.
BC has managed to achieve significant switching to biosimilars in just a short period of time through the recently enacted policy.
The program consisted of 2 phases, each containing a switching period during which both reference products and the respective biosimilars would be allowed to give patients time to discuss switching with their physicians. After the switching period concluded, BC PharmaCare would exclusively provide the biosimilar.
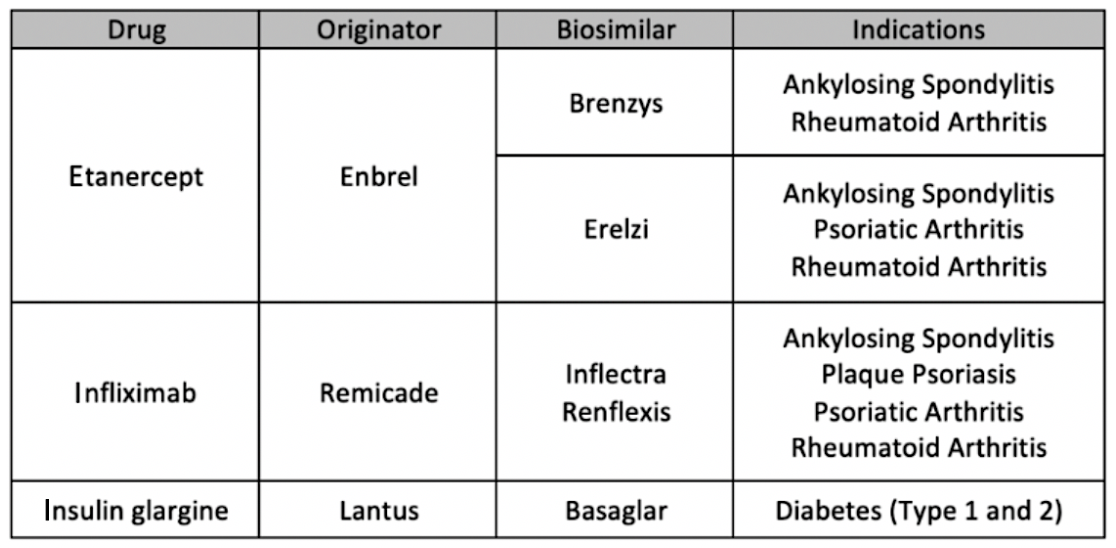
The first period lasted from May 27, 2019, through November 25, 2019, and patients were switched from 3 reference products: etanercept (Enbrel), infliximab (Remicade), and insulin glargine (Lantus), according the BC’s government website.
Patients using Enbrel were switched to either Brenzys or Erelzi depending on their condition. Patients using Remicade were switched to either Inflectra or Renflexis for certain indications. Lantus users were switched to Basaglar for patients with either type 1 or type 2 diabetes.
Overall, 15,100 (73%) of 20,780 users of reference biologics were switched to biosimilars during the first phase of the initiative. There were 1950 users of etanercept; 18,420, insulin glargine; and 410, infliximab. The respective numbers who switched to biosimilars were 1660 (85%), 13,100 (71%), and 340 (84%). Numbers on actual dollar savings were not provided.
Purchases of the 3 reference biologics involved are among the largest drug expenditures in BC, costing a total of $91.9 million in 2018.
Phase 2 lasted from September 5, 2019, through March 5, 2020, and expanded the indications allotted for switching from Remicade to Inflectra or Renflexis to include Crohn disease and ulcerative colitis.
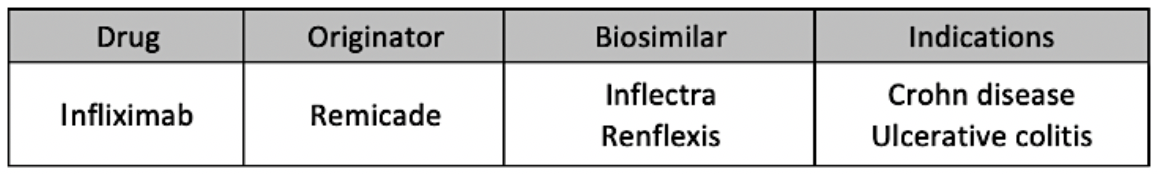
According to partial numbers on the second phase of the program, 78% of patients on infliximab originator product (N = 1860) were switched to biosimilars (1450).
“Although the first 2 phases are now over, the Biosimilars Initiative is an ongoing project and further switches to biosimilars are expected for other indications and drugs,” BC government officials said in a statement.
Although originator companies stand to lose revenue from forced switching policies, makers of biosimilars stand to benefit. In a recent statement, Sandoz Canada, which currently has 4 biosimilars approved, including a pegfilgrastim (Ziextenzo), rituximab (Riximyo), somatropin (Omnitrope), and etanercept (Erelzi), expressed support for the switching policy in BC.
Sandoz Canada stated that BC’s Biosimilar Initiative has the potential to generate significant cost savings for the BC health care system through a broader adoption of biosimilars based on some of the most expensive biologics on the market.
“It will…help accelerate the adoption of biosimilar beyond what is currently available. We urge other provinces to follow BC in taking the essential steps required to create a sustainable Canadian health care system,” said Michel Robidoux, president and CEO of Sandoz Canada, in a statement.
Similar to the United States, biosimilar approval in Canada does not automatically deem the product to be interchangeable with its reference product at the pharmacy level.
Although the United States requires additional clinical trials to establish interchangeability, in Canada, that authority is reserved for individual provinces based on their rules and regulations regarding pharmaceuticals.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
