- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
AAD Posters Examine Clinical Effects of Switching to Ustekinumab, Adalimumab Biosimilars
Two posters presented at the American Academy of Dermatology (AAD) annual meeting examined the effects of switching from reference ustekinumab and adalimumab to biosimilar versions in patients with different types of psoriasis.
Two posters presented at the American Academy of Dermatology (AAD) annual meeting examined the effects of switching from reference ustekinumab and adalimumab to biosimilar versions in patients with different types of psoriasis.
Image credit: SergeVo - stock.adobe.com
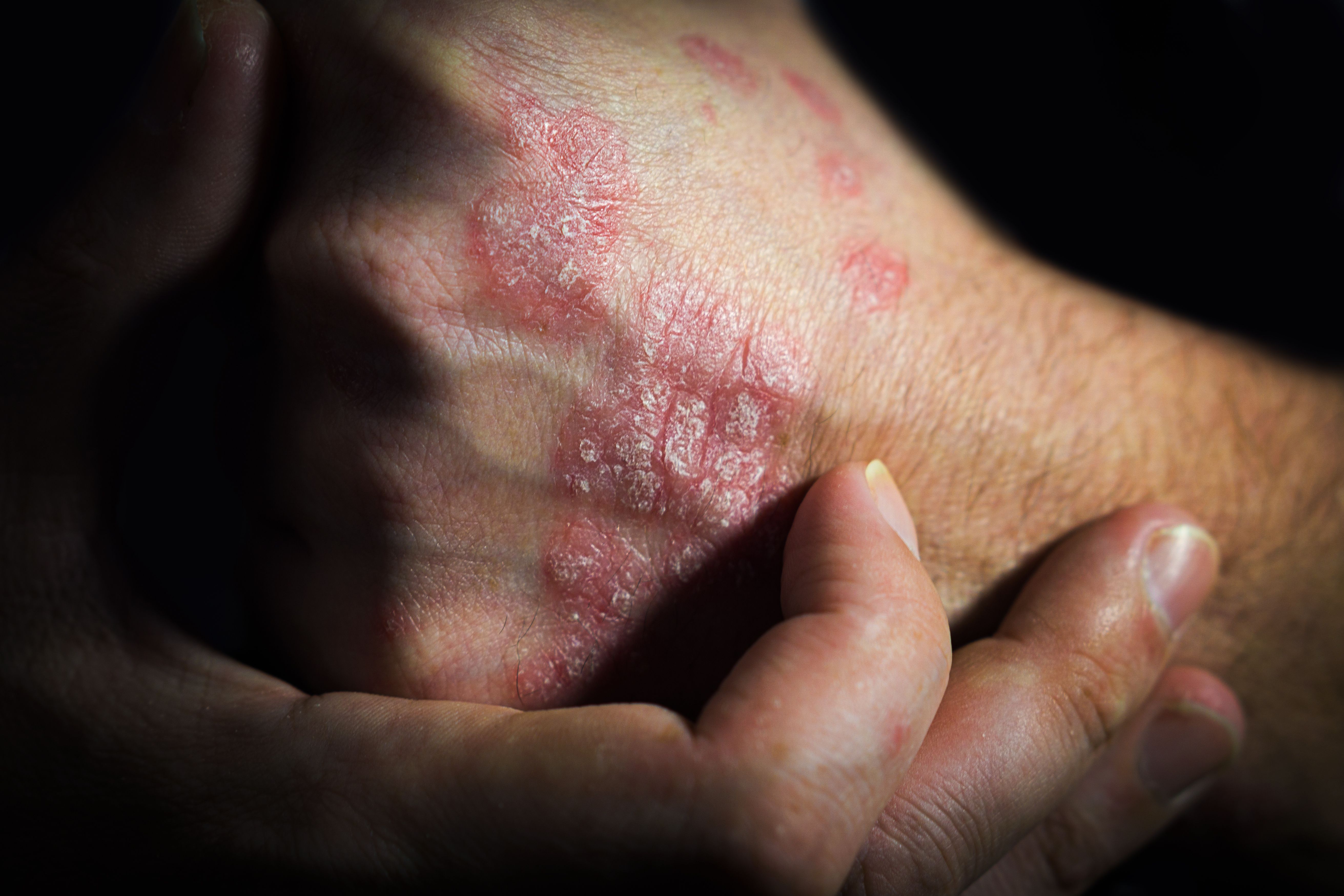
Ustekinumab Biosimilar in Plaque Psoriasis1
A randomized, double-blind, phase 3 study confirmed the safety and efficacy of switching from reference ustekinumab (Stelara) to SB17, an ustekinumab biosimilar candidate, in patients with moderate to severe plaque psoriasis.
The study included 503 patients. At baseline, patients were randomized to receive the 45 mg reference product (n = 254) or the biosimilar (n = 249) at weeks 0, 4, and every 12 weeks up to week 40. At week 28, patients were rerandomized to determine whether the patient should stay on their current product or switch. In total, 481 patients (SB17: n = 237; originator: n = 244) were rerandomized and 466 (SB17: n = 233; originator to SB17: n = 117; originator to originator: 116) completed 52 weeks of treatment.
Results showed that the percent change in Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI) scores up to week 52 were comparable between groups. Safety parameters were also similar across the board, with COVID-19 being the most common treatment-emergent adverse event (1.9%).
Although immunogenicity measures were lower in the SB17 group compared to the originator group, they were comparable between groups (SB17 to SB17: 16.5%; originator overall: 41.3%; originator to SB17: 39.3%; originator to originator: 43.4%). The occurrence of new onset overall antidrug antibodies after the transition was similar across treatment groups, with rates of 5.6%, 5.9%, 5.1%, and 6.7%, respectively.
Switching Patients From Reference Adalimumab to a Biosimilar2
Researchers assessed the safety properties associated with switching patients with psoriasis from reference adalimumab (Humira) to a biosimilar version (Imraldi), finding that many patients experienced an increase in adverse events after switching products.
The retrospective, observational study collected data from Belfast Health and Social Care Trust. Between 2019 and 2020, 106 patients with psoriasis who had been taking Humira for at least 12 months were examined. All patients were switched to Imraldi, 73 of whom stayed on the biosimilar and 31 switched back to the originator or Amgevita, another adalimumab biosimilar. Of those who failed treatment with Imraldi, 61% switched back to Humira and 39% switched to Amgevita.
The mean ages were 55 for the patients who switched back and 54 for those who remained on the biosimilar (control group). Both groups had more male patients than female patients. Additionally, patients in the switch back group had significantly higher percentages of high impact site involvement (control vs switch back: 48% vs 81%; P < .001).
Switch backs occurred mainly due to the relapse of psoriasis and injection site pain. Of those who switched back, 94% showed improvement, while 6% did not achieve satisfactory disease control and needed an alternative biologic agent. Before the initial biosimilar switch, the average PASI and DLQI scores were 0.6 and 0.3, respectively. These scores increased to 4.8 and 5.4 at the time of switch and decreased to 2 and 2.6 upon switching back.
Patients who did not respond well to biosimilar switching were more likely to be male, have a higher body weight, suffer from psoriatic arthritis, and have more extensive involvement of high-impact sites.
Although the poster did not mention it, other studies with similar results have attributed switch backs to the nocebo effect, where patients negative opinions about a medication result in adverse events.
References
1. Feldman S, Narbutt J, Girolomoni G, et al. Clinical similarity of SB17 (Proposed Ustekinumab Biosimilar) to reference ustekinumab in patients with moderate to severe plaque psoriasis: randomized, double-blind, phase iii, 52-week results. Presented at: AAD Annual Meeting; March 8-12, 2024; San Diego, CA. Poster 49104.
2. Elamin S, Attard M, McQuaid M, McKenna K. Outcome of psoriasis patients switched from adalimumab originator to biosimilar: a retrospective, observational study. Presented at: AAD Annual Meeting; March 8-12, 2024; San Diego, CA. Poster 50807.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
