
- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
AMCP Nexus: Panelists Share Current Scope of Biosimilar Industry
Panelists at the Academy of Managed Care Pharmacy (AMCP) Nexus meeting chronicled the current state of the US biosimilar market, including current policies impacting the market, recent regulatory decisions, and the developing arguments around requirements for clinical efficacy studies.
As the US biosimilars industry continues to grow and more products enter the market, a better understanding of the development process, regulatory framework, and policy landscape can assist pharmacists in propelling the industry forward, according to panelists at the AMCP Nexus meeting.
Chelsee Jensen, PharmD, BCPS, senior pharmacy specialist and pharmaceutical formulary manager at Mayo Clinic, began the session with an overview of the market, emphasizing the significant adoption growth since 2020, especially for products in the medical benefit (bevacizumab and filgrastim biosimilars). She also highlighted the current biosimilar pipeline, including several eculizumab, aflibercept, denosumab, and ustekinumab products as well as the recent FDA approval of the first tocilizumab biosimilar.
Jensen mentioned Mayo Clinic’s success with implementing biosimilars into regular practice, saving $23.1 million and $10.8 million in the program’s first and second years, respectively. She said that collaborating with payers, optimizing electronic health records, and communicating with prescribers in a clear and timely manner about upcoming changes are important strategies for health systems to take to ensure successful biosimilar implementation.
The outline of the regulatory framework for biosimilars was presented by Juwaria Waheed, MD, Scientific Reviewer for the Office of Therapeutic Biologics and Biosimilars. Waheed explained that the comparative analytical assessment comparing a biosimilar to its reference agent is the foundation of the biosimilar approval pathway. However, clinical studies play a small role in approval decisions because they are intended to demonstrate comparable efficacy and safety rather than establish new evidence.
“It is not a sensitive enough endpoint to tell you if there were actual differences in the product, we wouldn't be able to tell with that endpoint. So, it's a limited endpoint. It works great works excellent in de novo programs as well as for comparative efficacy. But if there were truly differences at an analytical level [between the products], it would be very hard to tell with these clinical studies.”
Waheed said that the FDA is working to further decrease reliance on clinical studies by leveraging analytical data and gaining more experience with biosimilars approved so far.
In addition to clinical efficacy trials, switching studies to obtain interchangeability are contentious. Jensen noted that study design is very important when evaluating whether transitioning from a reference product to a biosimilar and vice versa is safe and effective to do, quoting a study that found that cross switches that occurred within a 6 month or less period could hamper the ability to accurately trace immune responses.
As part of BsUFA III, the FDA funds "demonstration projects" to identify alternatives to comparative clinical immunogenicity assessments and leveraging real-world data as a means to enhance regulatory decision-making and facilitate science-based recommendations for biosimilar development.
Click to enlarge.
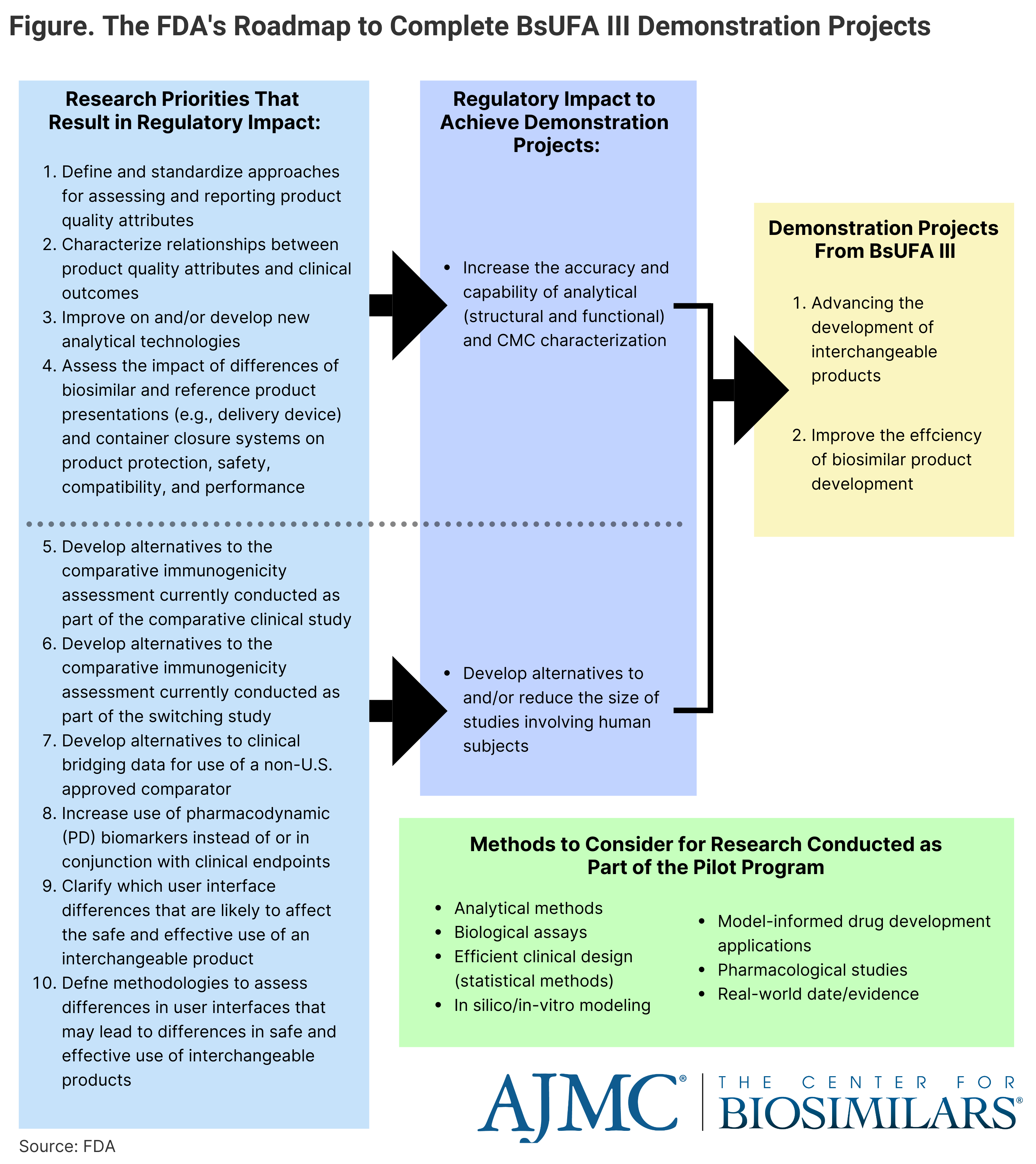
Kimberly Maxfield, PhD, a pharmacologist for the FDA, went over biosimilar marketplace trends and policy considerations, such as the impact of the third authorization of the Biosimilar User Fee Act (BsUFA III), which increases the FDA’s funding for projects designed to enhance regulatory decision-making and facilitating science-based recommendations in areas foundational to biosimilar development. BsUFA III extends from October 1, 2022 to September 30, 2027. (Figure)
The FDA has created a pilot program as part of BsUFA III, which will help find alternatives to reducing the size of clinical trials and increase reliance on analytical data through research projects. The program has funded several projects looking at analytical similarity, developing alternatives to comparative clinical immunogenicity assessments, and leveraging real-world data.
Maxfield concluded, “A lot of this program is defined by our stakeholders…We've tried to do as much stakeholder engagement as absolutely possible [with] actual user fee negotiations, public comment periods, and [including] broader non-FDA reviewers into the grant review cycle…. And we're still looking at other methods to make sure that the smaller developers, the less experienced developers who have different questions than the more experienced ones, are having their voices is be heard.”
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
259 Prospect Plains Rd, Bldg H,
Cranbury, NJ 08512
All rights reserved.