- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
American Rheumatology Network Promotes a Biosimilar Pathway
A pathway model that incorporated use of biosimilars has shown great promise, according to Colin C. Edgerton, MD, executive chairman of the American Rheumatology Network.
The American Rheumatology Network (ARN) has been so successful with cost management, particularly via the use of biosimilars, that it believes its model is worthy of emulation, said Colin C. Edgerton, MD, executive chairman of ARN, who spoke on the subject recently in an Academy of Managed Care Pharmacy—sponsored webinar.
“Certainly, rheumatologists are eager to see price reductions, which will lead to increased patient access to these critical medications for our patient population,” Edgerton said.
Edgerton practices with Low Country Rheumatology, a private practice in Charleston, South Carolina. The group used a pathway for treatment, employing biosimilars, that significantly lowered costs for patients.
Pathway Cut Costs by 70%
In 2016, the group enrolled 1867 patients in the pathway and 1001 patients off pathway. The costs, including all medical claims for on-pathway patients, was $8344, with estimated pharmacy spending of $5792, for a total of $14,136.
That compares with significantly higher costs for the off-pathway patients: $15,564 for all medical claims, $33,035 for pharmacy costs; and $48,599 in total (Figure). This represented a savings of 70%.
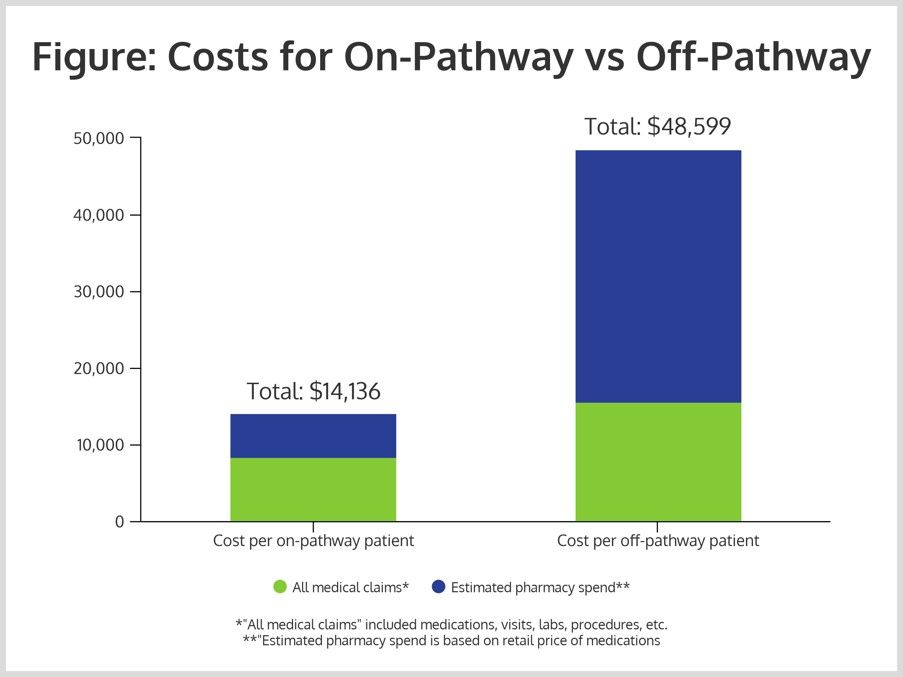
The model of care was constructed via discussions with providers and payers. The challenge was to convince all stakeholders that the use of biosimilars represented both savings potential and good care for patients. In some cases, payers had contractual agreements with originator drug manufacturers that represented barriers to biosimilar use. However, Edgerton has said it was possible to mitigate these circumstances by speaking a language that payers understood: biosimilars can represent lower costs and good care for patients.
The pathway model has been made available to independent practices represented by ARN.
Edgerton said that 65% of patients at his practice were on the pathway (n = 1867) and that by raising that by 5 percentage points, another $5 million in savings could be generated.
“It goes to show the real power you have when you intervene on the provider behavior in a way that adheres to national guidelines; a relatively small number of patients can lead to a very substantial savings,” Edgerton said.
Savings Matter in a Rising-Cost Environment
An estimated $120.1 billion was spent on biological medicines in 2017, up from $76.9 billion in 2013, according to a Forbes analysis. Edgerton believes the steady increase is unsustainable, especially in the rheumatology profession. “There's a palpable discomfort that rheumatologists feel as a small specialty with a bull's-eye on our backs, given the prescribing of these very expensive medications,” Edgerton said.
Additionally, rebates, step therapy, and prior authorization policies have led to price increases for patients.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
