- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
BioRationality: FDA Updates Its Biosimilars Action Plan—A Major Step in Bringing More Biosimilars to Patients
Sarfaraz K. Niazi, PhD, analyzes updates to the FDA's Biosimilars Action Plan, as well as the agency's increased efforts to boost biosimilar adoption and education.
The FDA has now approved more than 50 biosimilars, and the agency has taken a significant step in revising its 2018 Biosimilars Action Plan (BAP), intended to bring regulatory changes that will support a broader availability and adoption of biosimilars. This revision brings greater clarity to the FDA's thinking, but the most crucial element is a direction to developers and other stakeholders on how to increase the accessibility of biosimilars.
Over the past 5 years, the FDA has accomplished most of the goals in the 2018 BAP and released a 2018 Biosimilars Action Plan Summary Report to provide a detailed account of these deliverables and activities. With such accomplishment, the FDA has put the stakeholders back in line to promote biosimilars. It is time for the associations to shift their role and support the FDA rather than advertising biosimilars.
FDA is working to improve efficiency and predictability in the biosimilar and interchangeable biosimilar development and review process by refining review-related processes and practices and enhancing communication and consistency of development and review-related procedures. (Figure)
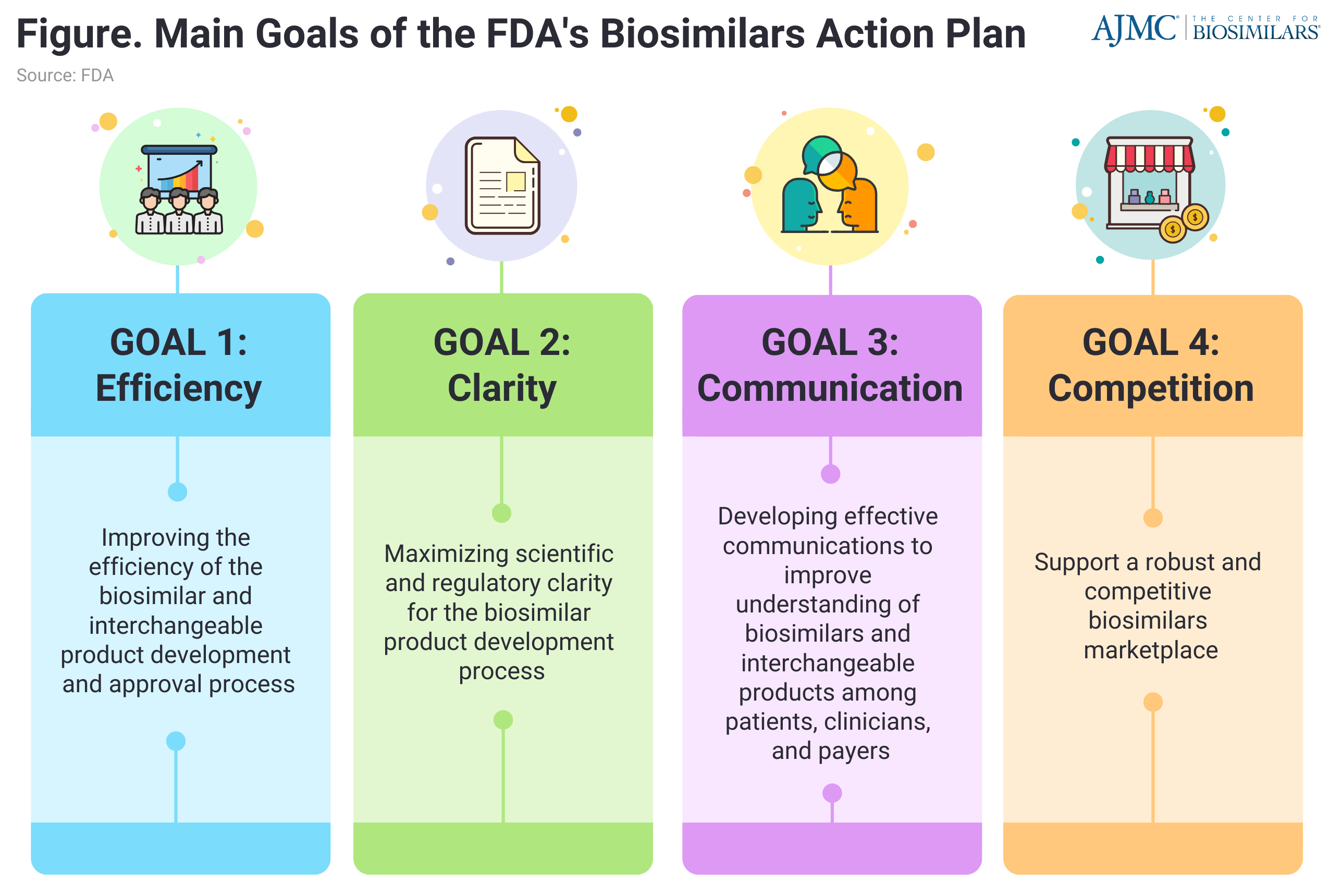
Streamline clinical development programs was accomplished through meetings such as the FDA/IPRP Workshop: Increasing the “Efficiency of Biosimilar Development Programs—Reevaluating the Need for Comparative Clinical Efficacy Studies”, FDA’s “Research in Biomarkers and Their Role in Biosimilar Product Development”, and maximizing scientific and regulatory clarity for the biosimilar product development community.
The Purple Book database contains information about all FDA-licensed biological products CDER regulates. It lists the patents involved in developing biosimilars and presents a significant action by the FDA.
Another significant support from the FDA is the recent guideline, “Labeling for Biosimilar and Interchangeable Biosimilar Products.” It specifies that the interchangeable status cannot be listed on the label and that innovators or developers may not promote their products based on any superiority claim.
FDA has also given out several Regulatory Research Pilot Program Research Awards and held a 2-part meeting to discuss the revised research roadmap and the direction of the Regulatory Science Pilot Program in October 2023.
The agency has also continued to collaborate with international regulatory authorities to facilitate the global development of biosimilars, as evidenced by its participation in the International Pharmaceutical Regulators Programme and chairing its Biosimilars Working Group and regularly meet with international regulatory authorities through the Biosimilars Cluster.
To develop effective communications to improve understanding of biosimilars and interchangeable biosimilars, the FDA is proactively educating clinicians, patients, and payers about biosimilar and interchangeable products.
FDA has developed educational materials for stakeholders, such as health care providers, patients, caregivers, advocates, payers, other government agencies, and the regulated industry. On March 2, 2023, the FDA and the Federal Trade Commission (FTC) released an educational resource for consumers about biosimilars and interchangeable biosimilars to help address common misperceptions, especially about interchangeable biosimilars.
- On March 21, 2023, the FDA released an updated infographic and 2 new fact sheets for patients in English and Spanish, including 1 for patients with diabetes
- On June 6, 2023, the FDA released 3 new animated videos for health care providers to learn more about biosimilars and interchangeable biosimilars
- On July 11, 2023, the FDA released a new animated video for patients
- Held a Reddit Ask Me Anything on the “r/pharmacy” forum to answer questions from the public about biosimilars and interchangeable products on June 7, 2023
- Conducted stakeholder outreach through webinars, presentations, and conference participation
- On March 2, 2023, the FDA and FTC released a report from the public workshop titled “Summary Report on the FDA/FTC Workshop on a Competitive Marketplace for Biosimilars.” This was to support the adoption of biosimilars, identify false or misleading statements about biosimilars, and deter anticompetitive behaviors in the biological product marketplace
While the FDA is taking an active role in identifying and counteracting misinformation about biosimilars and interchangeable biosimilar medications, resolving regulatory issues, and educating stakeholders, it is the stakeholders' responsibility to support the FDA's efforts. Notably, the FDA never mentions the biosimilar markets, a more common topic among many stakeholders; biosimilars are supposed to bring savings, but it is not news.
What should be news is that we will have more than just 15 molecules available as biosimilars in the US; the stakeholders should promote better regulatory guidelines and work towards harmonizing them globally to allow for better amortization of the development cost.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
