- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Canadian Experts Offer Switching Guidance for Biosimilars in Rheumatology
Preventing the nocebo effect requires a demonstration of confidence in biosimilars from all health care practitioners, including pharmacists, experts said in a newly published rheumatology treatment guidance document.
Rheumatology experts from Canada have developed pharmacist guidance for switching patients with rheumatologic disorders to biosimilars, which they hope also will be of use to the pharmacist community outside Canada.
The guidance was developed as Canadian provinces increasingly move toward mandatory switching to biosimilars to save money and improve access to care.
“New classes of antirheumatic drugs including janus kinase inhibitors (JAKs), along with the introduction of biosimilars are presenting more opportunity for therapeutic changes in patients with rheumatoid arthritis. These changes are often complex due to the nature of the drugs, along with an associated administrative burden,” they wrote. The group included physicians and pharmacists.
Consensus on Switching
In developing the guidance, the authors convened a panel of experts moderated by a facilitator and strived for consensus on key switching issues, primarily considerations prior to, during, and after transition to another biologic or JAK.
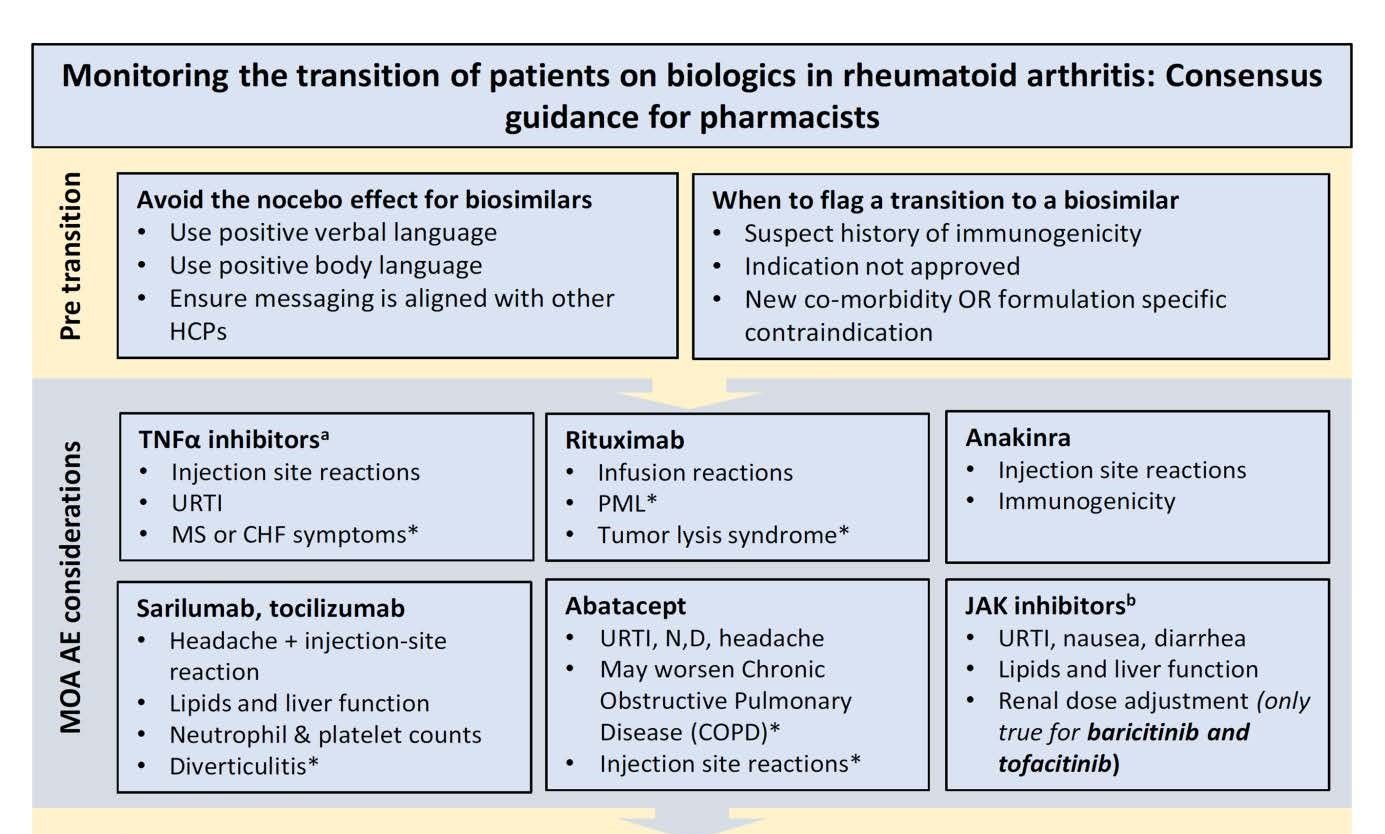
The panel paid close attention to the risks of the nocebo effect when switching therapies. Nocebo is when outcomes are affected by patient attitudes toward the therapy. The panel strongly recommended that pharmacists could best avoid nocebo by demonstrating confidence in the switch by using positive verbal language.
Consistent messaging from all involved health care providers was also seen as important to avoid this problem. “The participants did not see the value of using a validated tool to screen and assess patient attitudes toward the therapeutic transition as a proxy for potential nocebo,” authors of the guidance wrote.
Although biosimilars are not precise copies of originator drug products, and even originator biologics differ from batch to batch, scientific evidence has shown that “a single switch from a reference to a biosimilar is safe and effective. Additionally, clinical trials assessing switch from a bio-originator to a biosimilar have not demonstrated any loss of efficacy, increase in adverse events, or increased immunogenicity,” the authors wrote.
The group recommended that any concerns about potentially poor outcomes from a transition should be discussed with the prescribing rheumatologist prior to the switch.
Clinical Considerations
The authors provided a list of clinical considerations pharmacists should be aware of during a therapeutic switch. These were based on the mechanism of action for each therapeutic category. For example, with the use of tumor necrosis factor–alpha inhibitors, pharmacists should be aware of injection site reactions, upper respiratory tract infection, or symptoms of congestive heart failure.
After the transition, the best tool available to assess disease activity is a visual analog scale for the assessment of pain, fatigue, and quality of life, the group agreed. Following close behind in the ranking of utility were the RAPID3 (Routine Assessment of Patient Index Data 3) tool, which is a disease activity index based on a questionnaire, and the Likert Scale, which measures patient agreement with various prewritten statements.
Pain, fatigue, and quality of life are helpful in assessing for primary response, which is whether the patient has any initial benefit from the agent. The pharmacist is best positioned to monitor for these outcomes, the authors wrote, because the patient will likely interact with the pharmacist for refills prior to a follow-up appointment with the rheumatologist.
“Although a lack of peak-response early in treatment is not a particularly concerning, a series of uncontrolled or frequent flare ups as compared to baseline after 3 months of therapy would be cause for immediate referral,” they wrote.
Asked when a pharmacist should refer a patient to the rheumatologist for an earlier-than-scheduled follow up, the group emphasized the seriousness and severity of individual factors such as infection, repeated flare ups, and surgery; it said these should be the basis of such determinations.
“The pharmacist is well positioned to monitor and resolve issues relating to adherence and safety,” the authors wrote.
In their clinical considerations tool, the authors provided a “before starting” checklist to ensure a smooth transition to the substitute agent.
One of the questions considered by the panel but not addressed was how to measure the effectiveness of current therapy vs new therapy. “Participants felt it was inappropriate to attempt this as it may create the impression that we expect a change in effectiveness post-transition. This could lead to a nocebo effect,” the authors wrote.
Reference
Choquette D, Chan J, Bardi M, et al. Monitoring the transition of patients on biologics in rheumatoid arthritis: consensus guidance for pharmacists. Pharm Pract. 2021;19(3):2377. doi:10.18549/PharmPract.2021.3.2377
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.