- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Canadian Province Newfoundland and Labrador Adds Biosimilar Switching Policy
Newfoundland and Labrador is the latest Canadian province to implement a biosimilar switching policy, following in the footsteps of Ontario and 7 other jurisdictions.
Newfoundland and Labrador has become the latest Canadian province to implement a biosimilar switching policy requiring patients to be prescribed a biosimilar over a reference product, according to an announcement from Biosimilars Canada, a national association representing Canada’s biosimilar medicines industry.
“Biosimilars Canada and its member companies congratulate Health and Community Services Minister Tom Osborne and the Government of Newfoundland and Labrador for implementing a biosimilars switching policy to help support the long-term sustainability of the province’s public drug program,” Jim Keon, president of Biosimilars Canada, said in a statement.
The policy gives providers a until March 31, 2024, to prepare and educate patients currently treated on a reference product on the benefits of biosimilars to ease them into transitioning to a new product. New patients will automatically be prescribed a biosimilar. The policy will also allow the Newfoundland and Labrador Public Drug Program (NLPDP), the public health plan for the province, to invest in new and innovative therapies while continuing to expand access to existing drug therapies.
Under the initiative, beneficiaries currently using originator Copaxone (glatiramer acetate), Enbrel (etanercept), Humalog (insulin lispro), Humira (adalimumab), Lantus (insuling glargine), Lovenox (enoxaparin sodium), NovoRapid (insulin aspart), Remicade (infliximab), and Rituxan (rituximab) will be transitioned to a safe, effective and less costly biosimilar version. If a patient does not want to transition to a biosimilar, they risk losing coverage.
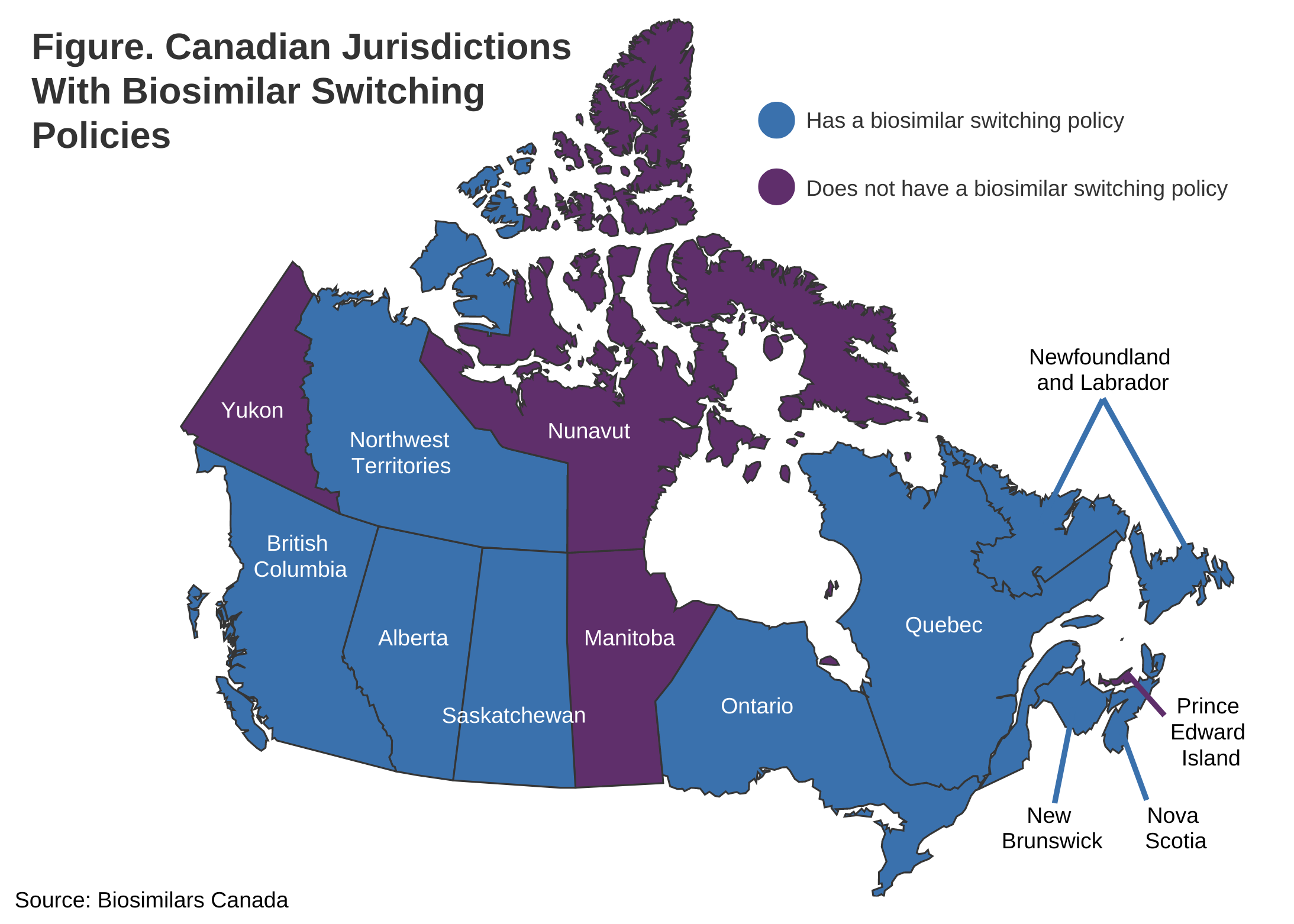
Newfoundland and Labrador is the ninth Canadian jurisdiction to implement a biosimilar transitioning policy, following British Columbia, Nova Scotia, New Brunswick, Saskatchewan, Quebec, the Northwest Territories, Alberta, and Ontario (Figure).
There are 9 Canadian jurisdictions with a biosimilar switching policy. Four (Nunavut, Manitoba, Yukon, and Prince Edward Island) do not.
The topic of switching or transitioning patients from a reference product to a biosimilar has raised concerns about whether product transitions will alter safety or efficacy outcomes. However, no province with a switching policy has experienced impacts on clinical outcomes and both the European Union and United Kingdom have declared all biosimilars as interchangeable with their reference products.
In its statement, Biosimilars Canada shared a quote from Health Canada’s biosimilar drug fact sheet on the safety of switching from a reference product to a biosimilar: “Patients and health care providers can have confidence that biosimilars are effective and safe for each of their authorized indications, and that no differences are expected in efficacy and safety following a change in routine use between a biosimilar and its reference biologic drug in an authorized indication.”
Biosimilars Canada commented saying that the full benefits of biosimilars, including their savings potential, won’t be realized unless drug plans adopt policies that support the expanded use of biosimilars, such as transitioning policies.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
