- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Celltrion’s Infliximab Biobetter Shows Significant Clinical Improvements Over Original Biosimilar Version
Results from the European Congress of Rheumatology’s annual meeting revealed that Celltrion’s Remsima SC, a subcutaneous biobetter infliximab product, produced better clinical outcomes compared with the original Remsima, an infliximab biosimilar delivered intravenously.
Results presented at the EULAR 2022 conference demonstrated that subcutaneous infliximab Remsima SC proved to have statistically significant improvement in clinical outcomes compared with the original intravenous formulation of Remsima.
EULAR 2022 is the European Congress of Rheumatology’s annual meeting. It is being held in Copenhagen, Denmark, from June 1 through June 4.
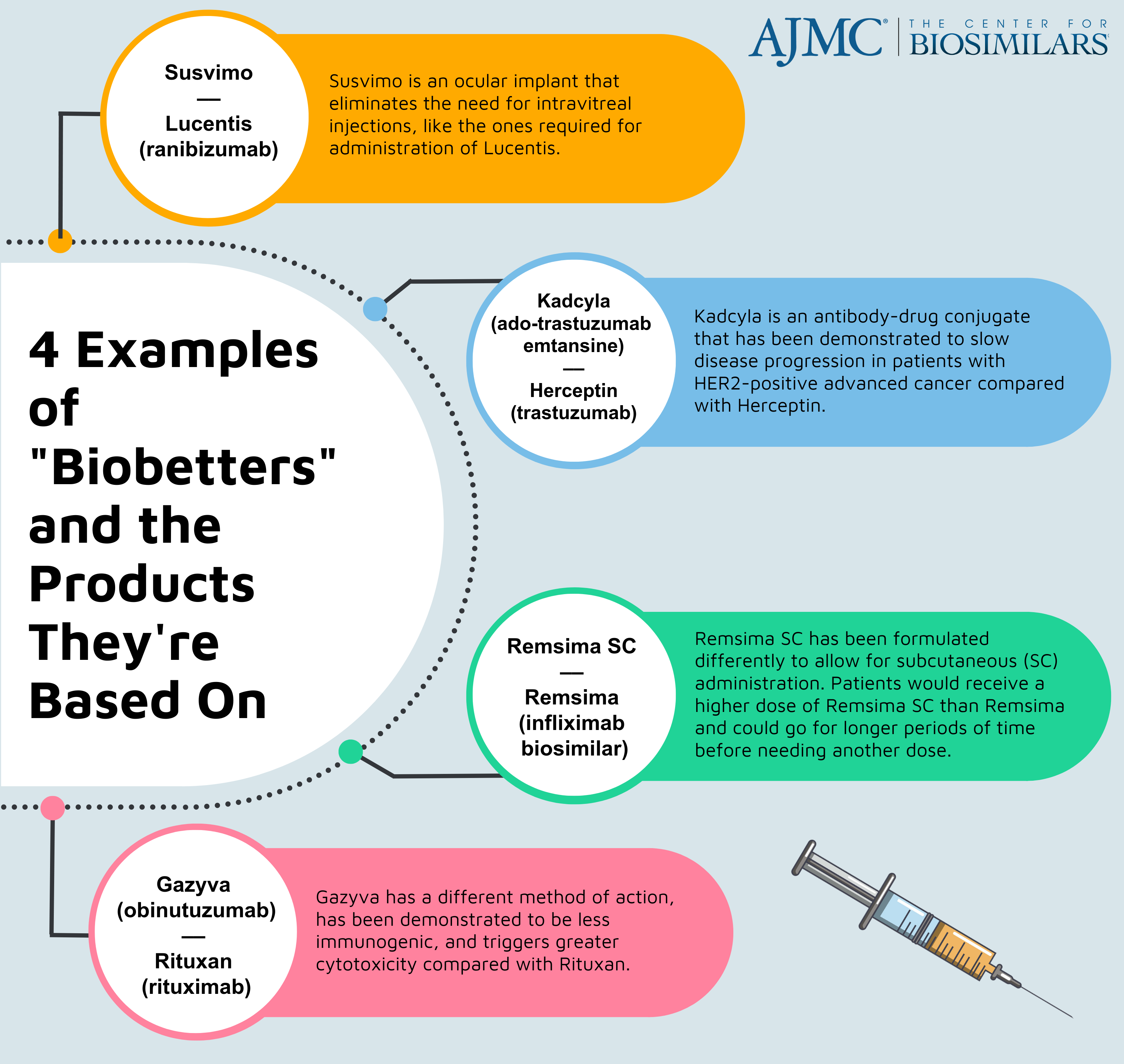
Remsima SC is a biobetter product drawing improvements on the original Remsima, an infliximab biosimilar referencing Remicade. Both products are priced lower than Remicade, the originator, but Remsima SC has been formulated to allow for subcutaneous administration and longer periods between doses. All 3 products are used to treat patients with rheumatic conditions, including rheumatoid arthritis (RA), psoriasis, and inflammatory bowel disease.
Click to enlarge
“Today, many medications have changed the treatment landscape of rheumatology, allowing low disease activity and remission to be achieved in people with RA.…Switching to medications such as the SC formulation of infliximab could allow patients to have better control of their treatment and improved quality of life,” said Arnaud Constantin, MD, PhD, a rheumatologist at Purpan University Hospital and a professor at Paul Sabatier University in Toulouse, France.
The new post hoc analysis of a phase 3 randomized controlled clinical trial shows that the Remsima SC was associated with lower disease activity rates and higher remission rates after week 30 and week 54.
The researchers utilized conservative imputation models, including nonresponder imputation and last observation carried forward. In total, 343 patients were randomized after receiving the Remsima biosimilar for 6 weeks to switch to a 120 mg dose of Remsima SC every 2 weeks (n = 165) or continue receiving 3 mg/kg of original Remsima every 8 weeks (n = 174).
Celltrion Healthcare, a Republic of Korea–based company, has a vast portfolio of biosimilars but has been increasing its focus on biobetters since 2019. Biobetters are a class of follow-on biologic products that are intentionally altered to improve clinical effects, allow for more time in between doses, or enhance tolerability. Although many biobetters are developed as alterations to originator biologics, Remsima SC is unique in that it is a biobetter based on a biosimilar.
Previous studies evaluating Remsima SC have shown that the biobetter produced superior clinical outcomes compared with the original infliximab biosimilar formulation in patients with inflammatory bowel disease, an umbrella term for Crohn disease and ulcerative colitis.
Remsima SC, also known as CT-P13, was approved for use for patients with rheumatoid arthritis in Canada in February 2021. The biobetter was also given approval by the European Commission for 5 indications (ankylosing spondylitis, Crohn disease, ulcerative colitis, psoriatic arthritis, and psoriasis) in July 2020.
In February 2022, Celltrion announced that sales for the original Remsima grew 13% year-over-year, amounting to $385 million, in the United States, where it is marketed under the name Inflectra. In April 2022, the original Remsima won Brazil’s tender to be the only infliximab product available to patients for a certain number of years.
Reference
Amneal achieves third U.S. biosimilar approval with Fylnetra™ (pegfilgrastim-pbbk). Press release. Amneal Pharmaceuticals; May 27, 2022. Accessed June 1, 2022. https://www.bloomberg.com/press-releases/2022-05-27/amneal-achieves-third-u-s-biosimilar-approval-with-fylnetra-pegfilgrastim-pbbk
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
