- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Celltrion Submits BLA for Infliximab Biobetter
Celltrion Healthcare submitted a biologics license application (BLA) to the FDA for its biobetter of its infliximab biosimilar allowing for subcutaneous administration (Remsima SC).
Celltrion Healthcare announced its submission of a biologics license application (BLA) for Remsima SC, a biobetter of the company’s infliximab biosimilar (Remsima) that allows for subcutaneous administration.
Intravenous Remsima was approved by the FDA in April 2016 and launched on the US market in November 2016, where it is marketed as Inflectra through a commercialization agreement with Pfizer. It is 1 of 4 infliximab biosimilars approved in the United States.
Remsima SC can be offered in a prefilled syringe or auto-injector pen
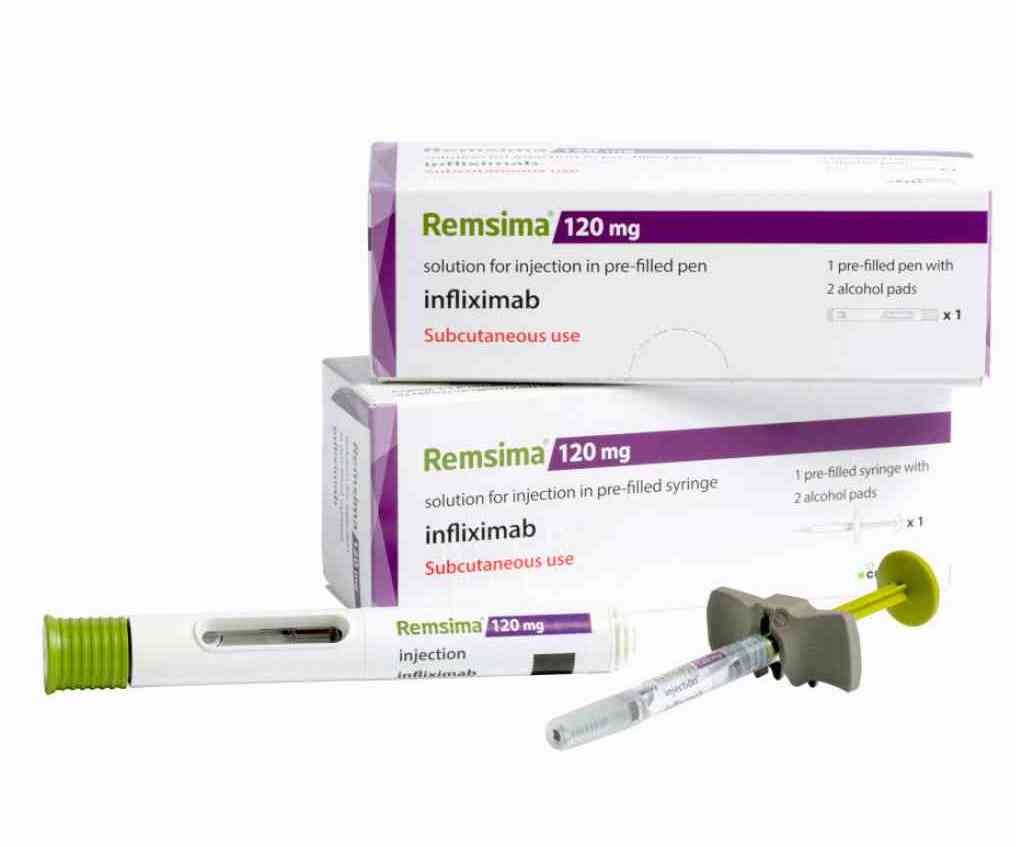
Remsima SC (CT-P13) is the world first subcutaneous infliximab product, which can be a more convenient method of administration for patients than intravenous injection. Additionally, the biobetter has been shown to produce better clinical outcomes compared with the original Remsima, according to data presented at the European Congress of Rheumatology’s annual meeting in June 2022.
The data from the phase 3 study demonstrated the safety and efficacy profiles of Remsima SC as maintenance therapy in patients with moderate to severe active ulcerative colitis (LIBERTY-UC) and Crohn disease (LIBERTY-CD). The results showed that Remsima SC had superiority over placebo in maintenance therapy after induction therapy of intravenous formulation infliximab in patients with UC and CD, respectively, over 1 year. The studies featured 438 patients with UC and 343 patients with CD. Both studies were randomized, placebo controlled, and double blinded.
“This BLA submission marks a significant milestone for Celltrion and we are working with the FDA to bring this innovative treatment to the U.S. market…. We are committed to furthering the advancement of innovative treatments that provide improvements to clinical outcomes and drug pharmacology and reduce patients’ burden on their day-to-day lives,” said Hyoung Ki Kim, vice chairman and CEO of Celltrion Healthcare, in a statement.
Biobetters are a class of follow-on biologic products that are intentionally altered to improve clinical effects, allow for more time in between doses, or enhance tolerability. Although many biobetters are developed as alterations to originator biologics, Remsima SC is unique in that it is a biobetter based on a biosimilar of Remicade (reference infliximab).
According to Celltrion, using Remsima SC can offer patients better consistency in drug exposure and allow for better drug adherence compared with intravenous administration.
“We’re excited about the potential of CT-P13 SC as it allows patients to have more control of their treatment, providing much better independence and convenience…. In addition, CT-P13 SC releases the burden of having to travel to treatment for IV infusions, reducing treatment-related travel costs for patients and caregivers,” commented Jaeik Shim, chief operating officer at Celltrion USA.
In February 2021, Health Canada approved Remsima SC for the treatment of rheumatoid arthritis. The product is also approved in the Republic of Korea and the European Union.
In an interview with The Center for Biosimilars® in August 2022, Kim said that he anticipates the FDA’s approval decision during the second half of 2023.
“Biobetters could be transformative for the biopharmaceutical space going forward—in the same way that the introduction of biologic therapies and biosimilars were….In light of advancing technologies and innovative approaches, biobetters could allow for improved patient and health care professional choice, and ready-to-use formulations such as Remsima SC could reduce the handling burden for clinicians, especially in light of the [COVID-19] pandemic.”
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
