- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
China Pulls Out Stops for Biosimilar Development
It took Henlius years to get a rituximab biosimilar approved in China. Now, that process could be cut in half, owing to significant regulatory reforms.
China has just 9 biosimilars approved vs 29 in the United States, and the United States is expected to stay ahead in this race through 2027. However, fundamental regulatory reforms in China have established a framework for biosimilar development and approval that will encourage robust competition in that market, according to a recent presentation by intellectual property (IP) attorneys from Goodwin Proctor.
“The regulation of research and development and the registration pathway for biosimilars in China will continue to become more structurally sound, clear, and transparent in the foreseeable years,” said Freddy Yip, PhD, PCLL, a Hong Kong–based attorney.
By US standards, there were once no true biosimilars in China, owing to lesser standards for approval, but regulatory policies have been tightened up considerably. In 2015, China created a standardized regulation for the development and evaluation of biosimilars, which Yip and Huiya Wu, JD, described as key to the biosimilars regulatory revolution in China. This clarified the definition of biosimilars and set standards for preclinical research and development, clinical trials, and manufacturing processes, creating what Yip said was the “foundation” for growth of China’s biosimilars industry.
In tandem with the various regulatory initiatives, China provided impetus to the biologics market by adding many biologics to the national reimbursed drug list (RDL). Up until 2017 this list included chemotherapy treatments but not oncology biologics, Yip said. “As a result, prices of these drugs decreased significantly, and as more biologics are included in the RDL, the affordability of biologics is expected to increase, which allows greater market access.”
Priority for Urgent Need
The country since 2018 has allowed priority review and approval for drugs that address urgent clinical needs and potential clinical trial exemptions for drug applications supported by robust clinical data from trials conducted overseas, Yip said.
Consequently, the China market for biologics has grown from $18 billion in 2014 to $40 billion in 2018, and the projection is for a $99 billion market to emerge by 2023, the attorneys said, citing Frost & Sullivan data. As in the United States, growth of the biologics market in China has broadly eclipsed the generics market expansion there. Yip predicted that in China, where there is much greater acceptance than in the United States for use of nonoriginator brands of medicine, the growth rate for biosimilars will soon “be much larger” than for their reference drugs.
In the United States, the biosimilars market in 2019 was $737.2 million vs $354 million in China. Strong growth is projected in both markets, but “it appears that the United States will continue to be the larger market through at least 2027,” Wu said.
It takes more time to get a biosimilar through the approvals process in the United States than in China, Wu said. Development time in China might take 5 years or more and cost upward of $50 million, vs 7 to 8 years and $75 million to $250 million in the United states, she estimated.
Another key characteristic that defines the China market for biosimilars is the dominance of relatively small biopharmaceutical companies, Wu said. In the United States, “for the most part, these are big-pharma, well-established companies, although there are smaller, newer companies, like Coherus BioSciences. There’s also a pretty strong representation by Republic of Korea companies like Celltrion.”
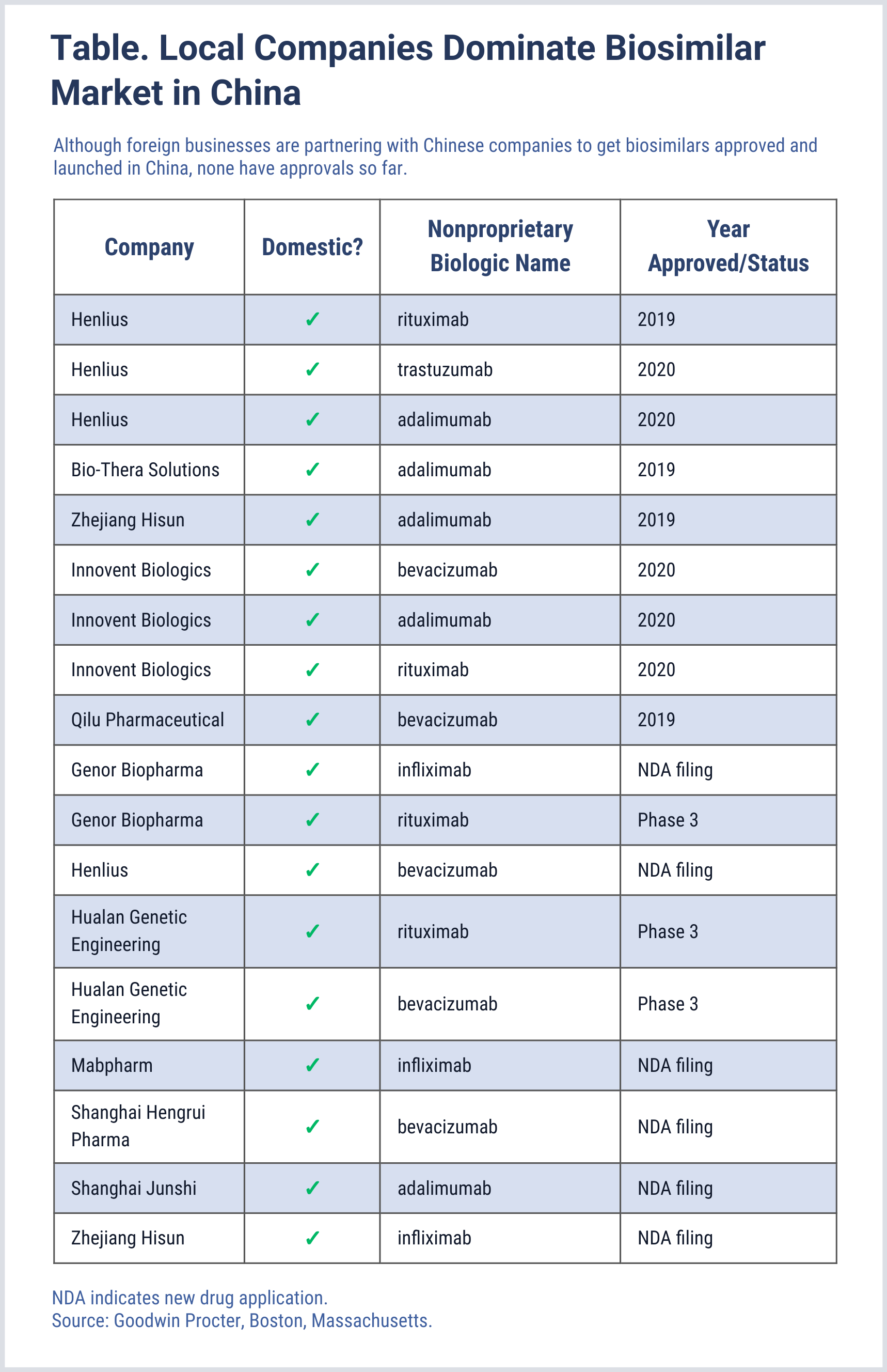
The China market may lack the representation of these large players, but its biosimilar companies have the advantage of intellectual property laws that may allow earlier entry of biosimilars. This has been the case for adalimumab (reference, Humira). The first adalimumab biosimilar in China was launched in January 2020 by Bio-Thera Solutions. The first US biosimilars for adalimumab are prevented from launching until 2023 owing to settlements between originator company AbbVie and biosimilar developers.
Although China-based companies hold all of the approvals so far for biosimilars in that market (Table), outside companies are partnering with China companies to gain the insight and leverage they need to penetrate that market and get a share of the growth. This includes Amgen, of Thousand Oaks, California, which has established a collaboration with China-based Simcere; and Samsung Bioepis, a Republic of Korea–based company working with China-based 3SBio. Celltrion hopes to build what will be China’s largest biologics manufacturing facility in Wuhan.
Click to enlarge
Shortened Review Times
China’s drug approval process has been significantly accelerated, Yip explained. Henlius began preclinical development for its rituximab biosimilar in 2010 and filed its investigational new drug application (IND) in 2011 (requesting permission to begin phase 1 in-human trials). It finally received IND approval from the National Medical Products Administration (NMPA) in 2014. In 2017 it filed a new drug application (NDA) with the NMPA and received NDA approval in 2019, becoming the first company to land an official biosimilar approval in China.
Today, that process would take substantially less time, depending on the quality of the application. “Each of the timeframes [from IND application to drug marketing authorization] is now measured in days, with the single longest step taking only 90 days,” Yip said. “For example, if an IND applicant does not receive any negative or questioning opinions from the NMPA within 60 days of the date of application acceptance and payment of the fee, clinical trials may be conducted in accordance with the plan that has been submitted.”
In 2018 and 2019 the Chinese government identified 78 priority drugs for which approval processes could be expedited and overseas clinical trial data used to support the applications. This was done to encourage pharmaceutical companies to bring these drugs to market as quickly as possible.
In general, Chinese regulators require in-country clinical trials that demonstrate drugs have been tested for safety and efficacy in Chinese populations. Now, “If there is no ethnic difference in the clinical study, sponsors can submit the clinical trial data obtained overseas and directly apply for the drug listing registration,” Yip said.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
