- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Common Patient Questions About Insulin Biosimilars Pertain to Classification
Nuances in classification for insulin biosimilars can lead to frequent areas of confusion, according to 2 pharmacists.
This was originally published in Pharmacy Times®. This version has been edited.
In the past decade, there have been many advances in the types of insulins available, including the emergence of biosimilar insulins. Since 2021, 2 biosimilar insulins have been approved, both of which are insulin glargine products and have interchangeability designations: Semglee and Rezvoglar. These biosimilar insulins provide additional options for patients with diabetes at lower cost; however, the nuances of biosimilar classifications and insulin biosimilar classifications are important to understand for appropriate dispensing. In particular, there are several common patient questions regarding these classifications that pharmacists may be asked.
Man injecting insulin | Syda Productions | stock.adobe.com

Is insulin a biological product/biologic?
The FDA defines a biologic product, or biologics, as a large molecule isolated from a natural source (human, animal, microorganisms), such as insulin, which is produced from bacteria or yeast. Although previously classified as a small molecule drug under the Federal Food, Drug, and Cosmetic Act, insulin is now considered a biological product.1
On the other hand, a biosimilar is a biologic that is very similar to an existing FDA-approved biologic agent, which is referred to as the reference product. The biosimilar drug must be without clinically meaningful differences in terms of safety, purity, and potency from its reference product. Further, all biologic products approved after 2015 have a 4-letter suffix after the product name to differentiate the from their reference product. Until more recent originator biologics with suffixes come off patent, the 4-letter suffix is easiest way to differentiate between a biosimilar and it's reference product (ex. adalimumab vs adalimumab-atto).2,3
Following the passage of the Biologics Price Competition and Innovation Act in 2010 by the US Congress, the FDA began working to devote additional resources to support the development of biosimilars. The first biosimilar product, a granulyte colony-stimulating hormone called filgrastim-sndz (Zarxio; Sandoz), was approved in 2015.4 Since then, the development of biosimilars has become an important way to bolster competition among manufacturers and decrease patient cost.5 This cost-saving opportunity is important for people with diabetes who require insulin, as the price of insulin has risen exponentially over the past 20 years. For context, 1 vial of insulin lispro (Humalog; Eli Lilly) was $21 in 1999. In 2019, that same vial cost $322.6
What biosimilar insulins are currently on the market?
In July 2021, insulin glargine-yfgn (Semglee; Mylan Pharmaceuticals) became the first biosimilar insulin and the first biosimilar to be approved as an interchangeable product. It references Lantus (Sanofi).7 A second interchangeable biosimilar insulin, insulin glargine-aglr (Rezvoglar; Eli Lilly), was approved in December 2021.8,9 It was granted interchangeability status in November 2022.
What is the difference between a biosimilar and an interchangeable biosimilar product?
A biosimilar cannot be substituted with its reference product without approval of the prescriber unless it is classified as interchangeable; when classified as such, state laws dictate whether the biosimilar can be dispensed for the reference product at the pharmacy without physician permission.
In most cases, to be approved by the FDA as an interchangeable biosimilar, the manufacturer of the biosimilar product must submit additional data to the FDA proving that the biosimilar product can be safely switched with the reference product without any meaningful changes in safety and efficacy.2 However, a new 2020 rule made it so that insulin products can be approved as interchangeable without switching data due to the long safety history associated with insulin products. Pharmacists should refer to the FDA Purple Book database of licensed biological products to determine whether a biologic product is interchangeable.10
Are interchangeable insulins cheaper than the reference product?
The approval of the 2 interchangeable insulin products has been shown to lower the cost of basal insulins. Table 1 shows the difference in average wholesale prices and patient costs from 2 companies (GoodRx and SingleCare) that offer discount cards for uninsured patients and illustrates the lower cost for consumers and pharmacies for the interchangeable products at the time the article was written.11-15
Table 1
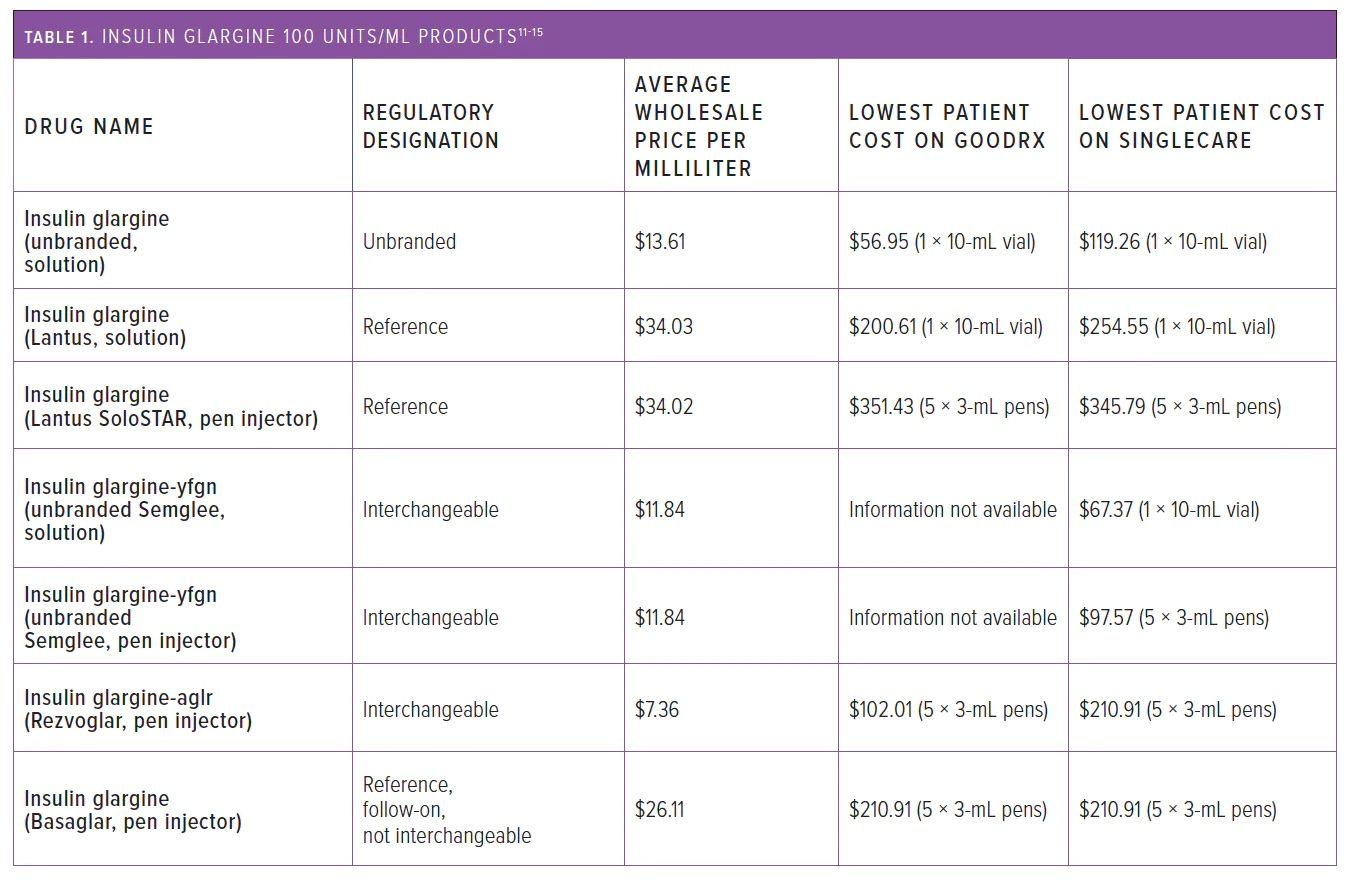
The list price for the consumer of the reference product, Lantus pen injectors (1 box = 5 Å~ 3-mL pens), was $345 to $352, whereas for branded insulin glargine-aglr, the cost was $98 to $102, and for unbranded insulin glargine-yfgn, the cost was approximately $98. The average wholesale price is also lower for the interchangeable biosimilars: It was $7.36 per mL for branded insulin glargine-aglr and $11.84 per mL for unbranded insulin glargine-yfgn compared with $34.02 per mL for Lantus pen injectors.16
Basaglar (Eli Lilly), another insulin glargine product, was approved by the FDA as a “follow-on” product instead of a biosimilar because it was approved before the establishment of the biosimilar approval pathway; it is classified as a biosimilar some international markets, including the European Union. Follow-on products are approved under the Federal Food, Drug, and Cosmetic Act 505(b)(2) under an abbreviated pathway and considered to be similar to the previously approved reference drug.
As of March 2020, Basaglar’s designation was changed to a biologic under the Public Health Service Act.1,14 However, it is currently classified as a reference product and is not interchangeable with Lantus.16 Despite this, Basaglar has similar outcomes on blood glucose and hemoglobin A1c to Lantus.17 Depending on state laws, pharmacists may need to contact the prescriber before substituting Lantus for Basaglar.15
As with any medication substitution, such as a switching from a bioequivalent generic for a brand drug or interchangeable biosimilar for a reference biologic, pharmacists should obtain approval from the patient, abide by state laws, and apply clinical judgment in determining whether a change should be made. To make informed decisions and provide accurate information to patients, pharmacists can utilize a variety of resources listed in Table 2 to broaden their knowledge and access up-to-date information and guidance on insulin biosimilars.3,10,18-20
Table 2
Are there any bolus/prandial/rapid/short-acting biosimilar insulins on the market?
At the time of publication, there are no bolus/prandial insulins that are FDA approved with any of the following reference products: insulin aspart (NovoLog, Novo Nordisk; Fiasp, Novo Nordisk), insulin lispro (Humalog; Eli Lilly), insulin lispro-aabc (Lyumjev; Eli Lilly), or insulin glulisine (Apidra; Sanofi). However, this could quickly change, as there are published clinical trials for a biosimilar insulin aspart and insulin aspart premix 70/30.20,21 Admelog (insulin lispro; Sanofi), a rapid-acting bolus/prandial insulin, was approved as a follow-on product and was changed in March 2020 and classified as a biologic product that is not interchangeable with insulin aspart.20-24
The European Union also has 3 insulin aspart biosimilars, 1 insulin lispro biosimilar, 1 human insulin biosimilar, and 3 insulin glargine biosimilars.
With More to Come, Stay Informed
Interchangeable biosimilars offer a lower cost for insulins and allow pharmacists to change a reference product to the interchangeable biosimilar without contacting the prescriber if the state laws allow substitution and the patient has given consent to make the change. Although there are currently only 2 interchangeable insulins on the market, more will become available. By being well informed, pharmacists can confidently navigate the realm of insulin biosimilars and ensure patients are receiving optimal diabetes care and able to access lower-cost insulin.
References
1. Drugs@FDA frequently asked questions. FDA. Accessed July 9, 2023. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=faq.page#nda_bla
2. Review and approval. FDA. December 13, 2022. Accessed July 9, 2023. https://www.fda.gov/drugs/biosimilars/review-and-approval
3. Overview for health care professionals. FDA. December 13, 2022. Accessed July 9, 2023. https://www.fda.gov/drugs/biosimilars/overview-health-care-professionals
4. FDA approves first biosimilar product Zarxio. FDA. March 6, 2015. Accessed July 9, 2023. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm436648.htm
5. Woodcock J. FDA. February 8, 2019. Accessed July 9, 2023. https://www.fda.gov/news-events/congressional-testimony/biosimilars-implementation
6. Rajkumar, SV. The high cost of insulin in the United States: an urgent call to action. Mayo Clin Proc. 2020;95(1)22-28. doi:10.1016/j.mayocp.2019.11.013
7. FDA approves first interchangeable biosimilar insulin product for treatment of diabetes. News release. FDA. July 28, 2021. Accessed July 9, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-interchangeablebiosimilar-insulin-product-treatment-diabetes
8. FDA roundup: November 18, 2022. News release. FDA. November 18, 2022. Accessed July 9, 2023. https://www.fda.gov/news-events/press-announcements/fda-roundup-november-18-2022
9. Rezvoglar becomes second interchangeable insulin biosimilar. Center for Biosimilars. November 23, 2022. Accessed July 9, 2023. https://www.centerforbiosimilars.com/view/rezvoglar-becomes-second-interchangeable-insulin-biosimilar
10. FDA Purple Book. FDA. Accessed July 9, 2023. https://purplebooksearch.fda.gov/
11. Biosimilar product information. FDA. Updated May 25, 2023. Accessed May 11, 2023. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information
12. PDQ: NCI’s Comprehensive Database. National Cancer Institute; 2015. Updated July 17, 2015. Accessed March 16, 2016. http://www.cancer.gov/publications/pdq
13. GoodRx. Accessed July 6, 2023. https://www.goodrx.com/
14. SingleCare. Accessed July 6, 2023. https://www.singlecare.com/
15. White J, Goldman J. Biosimilar and follow-on insulin: the ins, outs, and interchangeability. J Pharm Technol. 2019;35(1):25-35. doi:10.1177/8755122518802268
16. Basaglar. FDA Purple Book. Accessed July 9, 2023. https://purplebooksearch.fda.gov/results?query=insulin%20glargine&title=Basaglar
17. Overview of biologics and biosimilar products. American Pharmacists Association. 2021. Accessed July 9, 2023. https://www.pharmacist.com/Advocacy/Issues/Biosimilars/
18. Biosimilars toolkit. Biosimilars Council. 2023. Accessed July 9, 2023. https://biosimilarscouncil.org/biosimilars-toolkit/
19. Curriculum materials for health care degree programs: biosimilars. FDA. December 13, 2022. Accessed July 9, 2023. https://www.fda.gov/drugs/biosimilars/curriculum-materials-health-care-degree-programs-biosimilars
20. Newsome C. Basaglar. Clin Diabetes. 2017;35(3):181.doi:10.2337/cd017-0025
21. Blevins TC, Raiter Y, Sun B, et al. Immunogenicity, efficacy, and safety of biosimilar insulin aspart (MYL-1601D) compared with originator insulin aspart (Novolog) in patients with type 1 diabetes after 24 weeks: a randomized open-label study. BioDrugs. 2022;36(6):761-772. doi:10.1007/s40259-022-00554-6
22. Aravind SR, Singh KP, Aquitania G, et al. Biosimilar insulin aspart premix SAR341402 mix 70/30 versus originator insulin aspart mix 70/30 NovoMix 30) in people with diabetes: a 26-week, randomized, open-label trial (GEMELLI M). Diabetes Ther. 2022;13(5):1053-1071. doi:10.1007/s13300-022-01255-7
23. FDA approves Admelog, the first short-acting “follow-on” insulin product to treat diabetes. News release. FDA. March 24, 2020. Accessed July 9, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-admelog-first-short-acting-follow-insulin-product-treat-diabetes
24. Admelog. FDA Purple Book. Accessed July 9, 2023. https://purplebooksearch.fda.gov/results?query=insulin%20lispro&title=Admelog
About the Authors
Camlyn Masuda, PharmD, BCACP, CDCES, is an associate specialist of pharmacy practice at the Daniel K. Inouye College of Pharmacy at the University of Hawai’i at Hilo.
Chase Ibia and Rosalind Wong are PharmD candidates at the Daniel K. Inouye College of Pharmacy at the University of Hawai’i at Hilo.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
