- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Data From HERITAGE Confirm PK Biosimilarity for Ogivri
Pharmacokinetic (PK) parameters matched up with those of reference trastuzumab in an extended analysis of data from the HERITAGE trial.
Investigators have reported pharmacokinetic (PK) findings that confirm the biosimilarity of Viatris’ trastuzumab biosimilar candidate (MYL-14010, Ogivri) to reference Herceptin. Ogivri, developed by Mylan, which is now Viatris, was approved by the FDA in 2017.
The data come from the HERITAGE randomized phase 3 study, which previously demonstrated comparable efficacy and safety for Ogivri vs Herceptin as frontline treatment for women with human epidermal growth factor receptor 2 (HER2)–positive advanced breast cancer. The biosimilar was launched in the United States in December 2019, and these latest findings were presented at the 2020 San Antonio Breast Cancer Symposium (SABCS).
Patients (N = 485) were randomized to receive the biosimilar (n = 245) or reference product (n = 240) in combination with a taxane (docetaxel or paclitaxel) every 3 weeks for 24 weeks for 8 cycles, followed by additional cycles of monotherapy until disease progression, discontinuation, or unacceptable toxicity.
Primary Objectives of the Study
Primary objectives of the study were to compare drug concentration variation (area under the curve), maximum serum drug concentration, clearance (drug elimination), drug propensity to leave the plasma (volume of distribution), and terminal elimination half-life (no further drug distribution or absorption).
The investigators also sought to assess shed extracellular domain (ECD) HER2 receptor fragments and their effect on PK parameters. The decrease of shed ECD HER2 fragments is associated with an improved prognosis in metastatic breast cancer.
They took PK samples from patients at cycles 1, 2, 4, 6, 8, and 9 (monotherapy) to assess minimum serum concentrations. Serum maximum concentration samples were collected at the end of cycles 1 and 6. Additional samples were collected from a population subset. The final analysis included 213 patients in the biosimilar group and 202 in the Herceptin group.
No notable differences in demographics were observed between the 2 patient cohorts, the investigators said, noting there were no dissimilarities in trastuzumab concentrations between the groups. Treatment (biosimilar or reference) was not a significant covariate of clearance (P = .177) or drug distribution (P = .584).
However, ECD HER2 concentration was a significant factor in trastuzumab clearance, and weight was also a significant covariate of clearance and drug distribution (volume of the central compartment).
In the trough concentration (lowest drug concentration before the next dose) analysis, data were comparable between treatment arms at the end of cycles 1 and 6, the investigators said (Table).
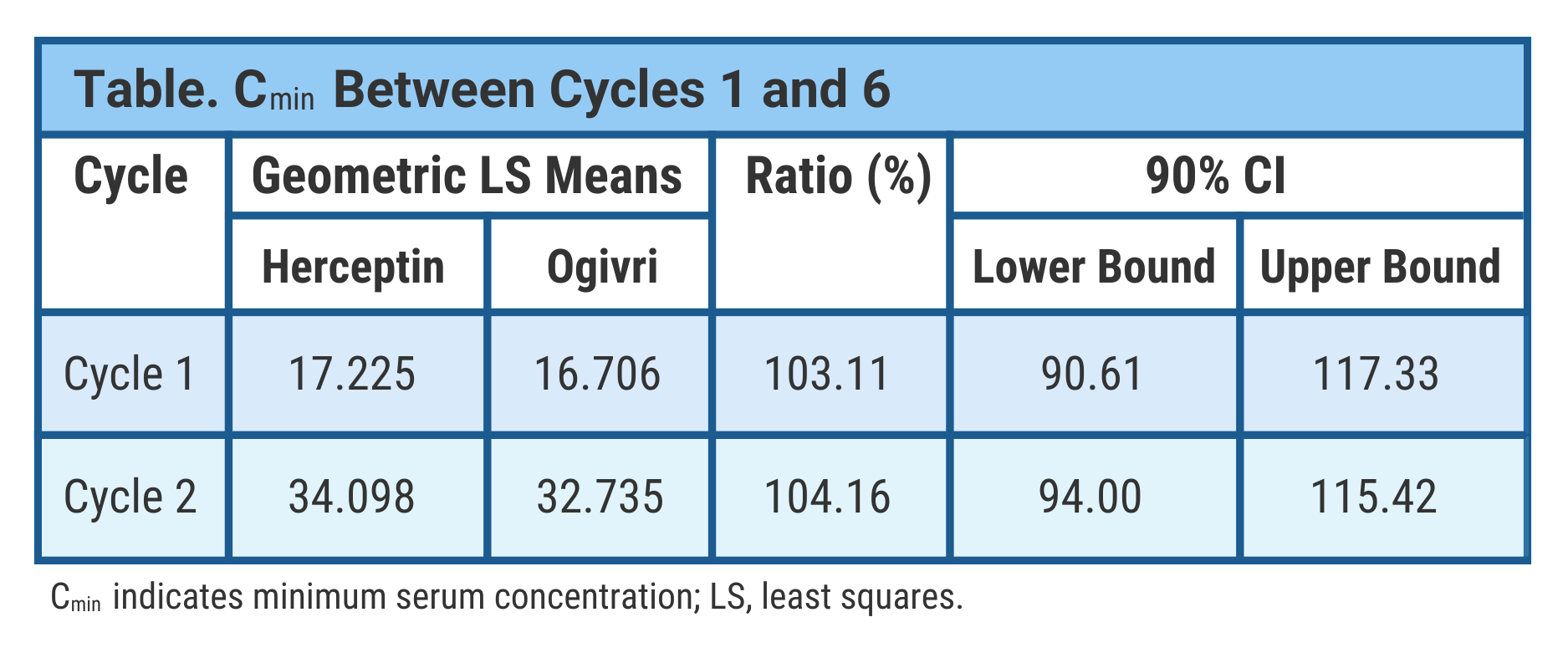
They used Bayesian statistics to evaluate steady state drug exposure between treatment arms and found comparable results indicating PK biosimilarity between the biosimilar and reference drugs. Steady state represents equivalent rates of drug absorption and elimination.
A low frequency of antidrug antibodies, which counteracts the effects of trastuzumab, were observed between Ogivri (n = 14) and Herceptin (n = 21).
Investigators concluded the population PK profiles of Ogivri vs Herceptin were comparable in patients with HER2-positive metastatic breast cancer. They said the study confirmed earlier PK findings.
Financial support for the study and its presentation at SABCS was provided by Viatris, investigators said.
Reference
Owen J, Rackley R, Liu M, et al. Population pharmacokinetics of MYL-14010 (a trastuzumab biosimilar) and reference trastuzumab (Herceptin) in patients with HER2-positvie metastatic breast cancer. Poster presented at: SABCS; December 8-11, 2020. PS18-23. https://sabcs.onlineeventpro.freeman.com/single-file-viewer/25581567
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
