- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Dutch Study Confirms Response, Survival for Rituximab Biosimilars in DLBCL
A retrospective study of rituximab biosimilars Truxima and Riximyo demonstrated comparable response and survival for patients with diffuse large B-cell lymphoma (DLBCL).
Using data from a population-based cancer registry in the Netherlands, investigators found similar response and survival rates for patients with diffuse large B-cell lymphoma (DLBCL) who received either rituximab originator (Rituxan) or biosimilars. They found that 3-year overall survival (OS) was 73% in both the originator and biosimilar groups (P = .855), and overall response rates (ORRs) were 85% in the originator group and 84% in the biosimilar group (P = .326).
Rituximab, a monoclonal antibody to the B-cell surface antigen CD20, has been “the backbone of every treatment regimen in B-cell non-Hodgkin lymphomas” since its approval by the FDA in 1997 and European Medicines Agency (EMA) in 1998, the authors said. And in the Netherlands, rituximab biosimilars were rapidly incorporated into the treatment regiment for these cancers.
Two rituximab biosimilars, CT-P10 (Truxima; Celltrion) and GP2013 (Riximyo; Sandoz), were approved by the EMA in 2017. That year, the Dutch Association of Medical Specialists began to incorporate rituximab biosimilars into the standard of care. By 2018, the authors said, 91% of the rituximab purchased in the Netherlands was biosimilar product rather than originator, leading to a 43% reduction in annual expenditure on rituximab in 2019 compared with 2015.
The investigators noted that rituximab biosimilars were approved by the EMA based on studies in patients with rheumatoid arthritis and naïve follicular lymphoma. Until now, data on ORR and OS rates with rituximab biosimilars in DLBCL, they said, have been “limited to small postmarketing studies.”
Investigators identified 4429 patients from the Netherlands Cancer Registry with DLBCL who were 18 or older, received a diagnosis of DLBCL between 2014 and 2018, and received at least 1 cycle of R-CHOP (a combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone), which they noted is standard of care for patients with newly diagnosed DLBCL. There were 3553 patients treated with rituximab originator and 876 treated with a biosimilar. Of those treated with a biosimilar, 86% received CT-P10 and 14%, GP2013.
Similar Response and Survival Rates
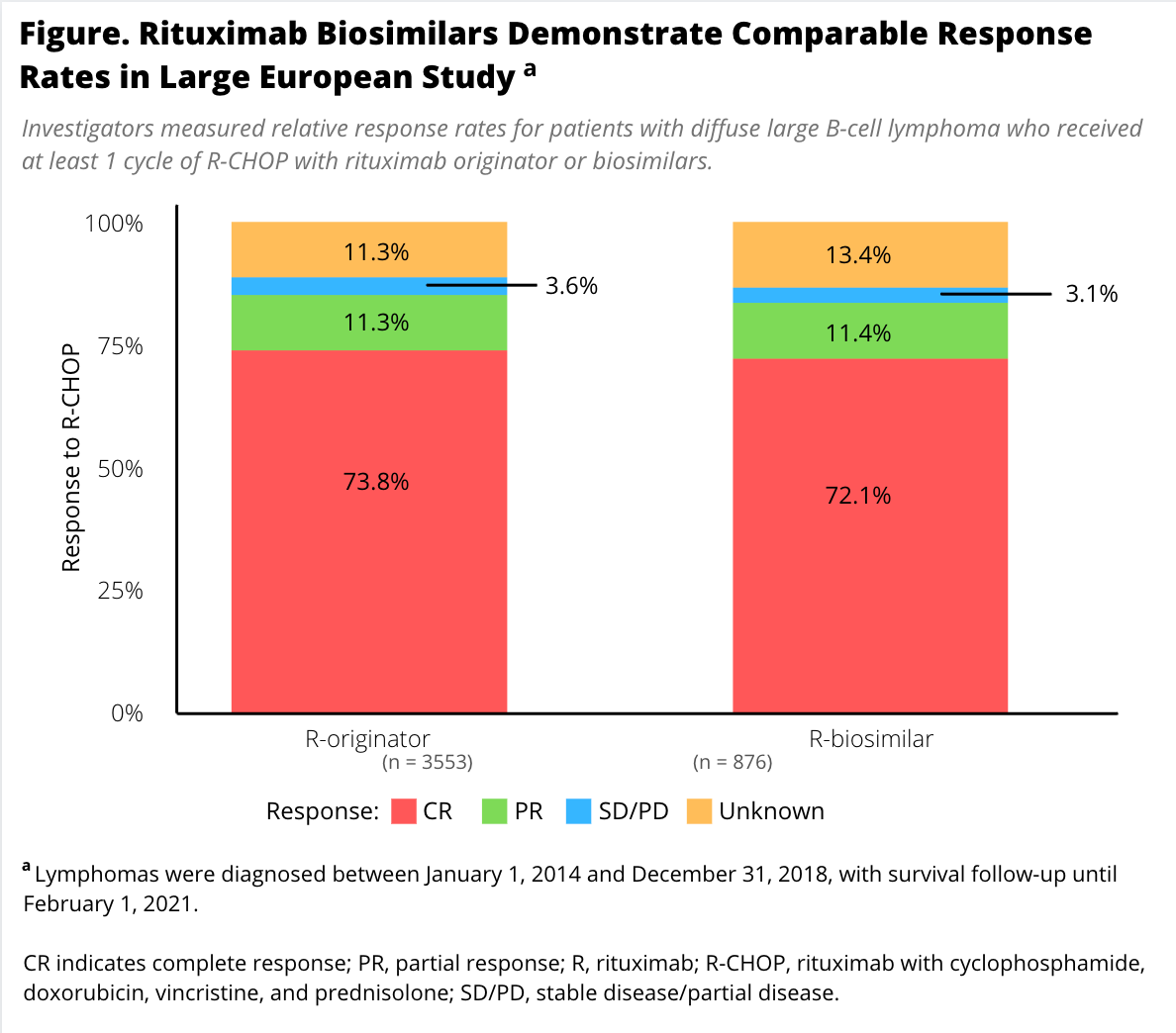
Complete response was achieved by 74% of patients in the reference cohort and 72% in the biosimilar group; 11% in each cohort achieved partial response (Figure).
Overall 3-year survival was 73% in each group. The risk of mortality was also similar between groups in a multivariate analysis that adjusted for age at diagnosis, sex, International Prognostic Index score, and number of cycles of R-CHOP in the biosimilar group (HR 0.89; 95% CI, 0.76-1.05; P = .182) compared with the originator group.
Sensitivity Analysis
Because the study included patient data from several years prior to when rituximab biosimilars were routinely used in clinical practice, the median follow-up times were 62 months for the reference product and 32 months for the biosimilars. The authors used sensitivity analysis to account for this difference.
First, an analysis of 3-year overall survival was conducted excluding all patients treated with the reference product who were diagnosed prior to biosimilar approval. Analyzing just the 370 patients treated with the reference product and 876 patients treated with biosimilars who were diagnosed between April 2017 and December 2018, 3-year survival rates were similar, 74% and 73%, respectively (P = .798).
In the second part of the sensitivity analysis, the researchers compared 3-year survival rates in patients receiving the originator before (n = 3183) and after (n = 370) biosimilar approval. They found 3-year survival rates of 73% for those diagnosed prior to April 2017 and 74% for those diagnosed afterward. “Overall, the results of the sensitivity analyses were similar to those of the primary analyses,” the authors wrote.
According to the authors, theirs is the first population-based study following EMA approval of the first rituximab biosimilar to investigate survival in patients with newly diagnosed DLBCL treated with the rituximab originator or biosimilar. They wrote that their data suggest that patients with DLBCL “benefit equally” from rituximab originator or biosimilars combined with CHOP treatment. They acknowledged “the lack of detailed information on dosage per cycle at the patient level and treatment-related toxicities, including infusion-related reactions,” as limitations of their study.
Reference
Brink M, Kahle XU, Vermaat JSP, et al. Impact of rituximab biosimilars on overall survival in diffuse large B-cell lymphoma: a Dutch population-based study. Blood Adv. 2021;5(15):2958-2964. doi:10.1182/bloodadvances.2021004295
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.