- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
FDA Approves First Subcutaneous Infliximab Product
The FDA approved Celltrion’s Zymfentra (infliximab-dyyb), a novel drug that allows for subcutaneous administration of inflximab.
Zymfentra (subcutaneous infliximab product) is marketed as Remsima SC in the European Union, Brazil, and the Republic of Korea.
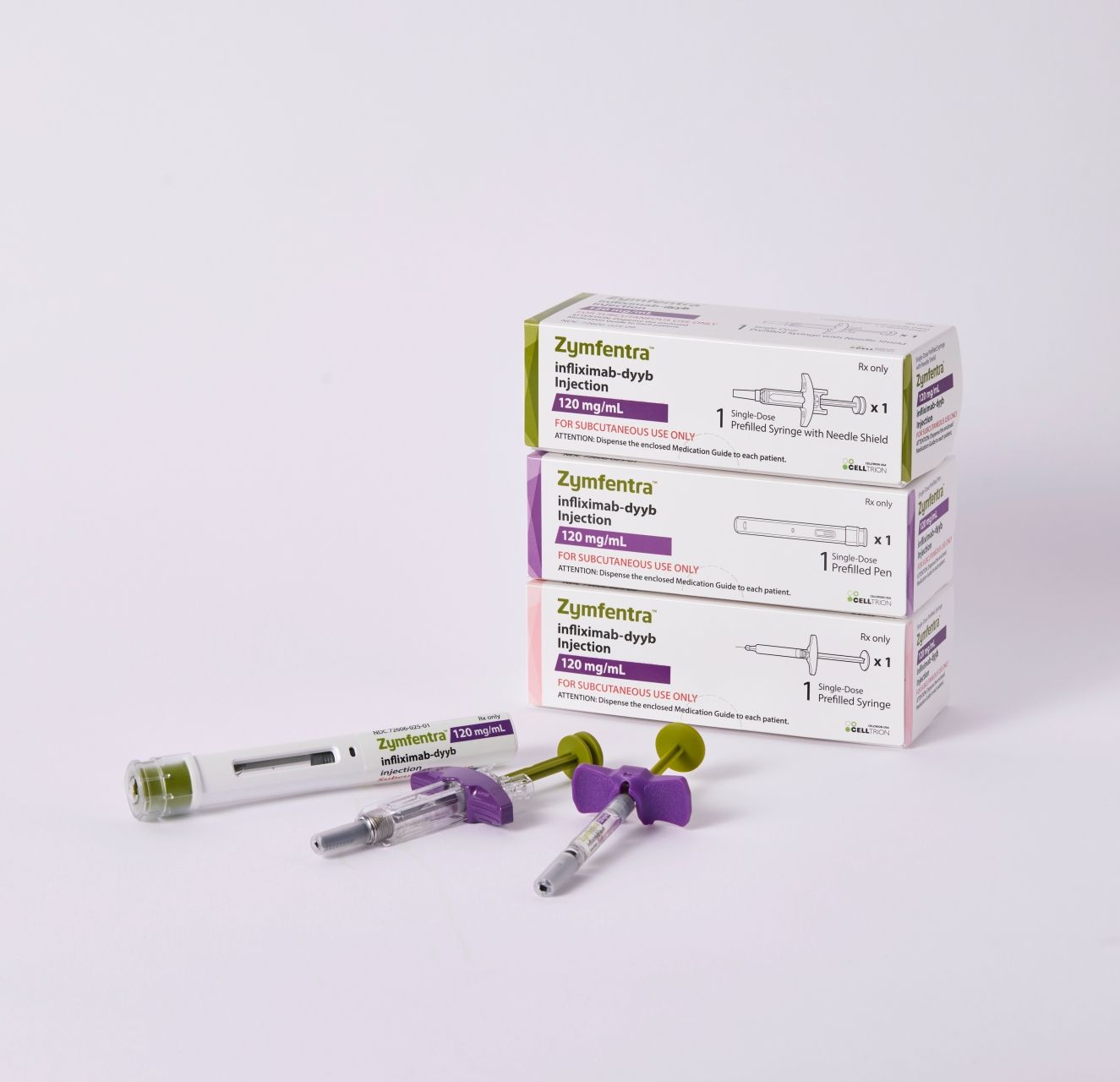
The FDA approved Celltrion USA’s Zymfentra (infliximab-dyyb), the world’s first and only subcutaneous infliximab product. Zymfentra is approved as a novel drug, and its development is based on Remicade (reference intravenous infliximab).
The intravenous version of Zymfentra is marketed as Inflectra (also, infliximab-dyyb), Celltrion's infliximab biosimilar. Inflectra was approved in April 2016.
Zymfenra was approved for the treatment of adults with moderate to severe inflammatory bowel disease (IBD)—an umbrella term for ulcerative colitis (UC) and Crohn disease (CD)—following treatment with an intravenously-administered infliximab product.
“There remains an unmet need for patients who suffer from the day-to-day burden of living with moderately to severely active Crohn’s disease and ulcerative colitis,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA, in a statement. “The approval of Zymfentra provides an innovative and effective treatment option that offers patients with IBD an alternative administration option providing control of how and where they receive their treatment, reinforcing our commitment to providing high-quality and affordable treatment options that deliver substantial value to patients and our healthcare system.”
Following the submission of the BLA under the 351(a) pathway of the Public Health Services Act, the FDA based its decision on data from 2 phase 3 pivotal trials that assessed the efficacy of Zymfentra as maintenance therapy in patients with moderate to severe UC (LIBERTY-UC) and CD (LIBERTY-CD). The data was presented at the European Crohn’s and Colitis Organization congress.
The results of studies shows that Zymfentra had a superior response in achieving clinical remission (UC and CD) and endoscopic response (CD) compared to placebo as maintenance therapy after induction therapy of intravenous infliximab over a 54-week period. The overall safety profile of Zymfentra was similar to that of placebo during the maintenance period in both studies, and no new safety signals were identified.
“As a healthcare professional dedicated to improving the lives of patients with IBD, I am excited to see further data that validate a convenient treatment option that could allow more patients in the U.S. to have greater control of their disease management,” said Jean-Frederic Colombel, MD, a gastroenterologist and professor of medicine at the Icahn School of Medicine at Mount Sinai.
What is a Biobetter?
In international markets, Zymfentra is considered a biobetter of Remsima/Inflectra because it has been formulated to allow for subcutaneous injection. However, biobetter is not a common term in the United States and Zymfentra has been approved as a novel drug Inflectra because it has been shown to perform better than placebo in clinical studies rather than shown to perform better than Inflectra or Remicade.
Biobetters are a class of follow-on biologic products designed to improve clinical effects, allow for more time in between doses, or enhance tolerability compared with their reference product. While the term “biosimilar” is applied to a drug that has been demonstrated to be highly similar to its reference, with no clinically meaningful differences from the originator product, the term “biobetter” refers to a therapy that has resulted from intentionally altering a biologic product in order to improve clinical outcomes.
Zymfentra—also marketed as Remsima SC in some international markets (European Union, Brazil, Republic of Korea)—is unique as a biobetter in that it is the only biobetter that improves upon a biosimilar rather than an innovator product. The approval comes about 9 months after Celltrion submitted the biologics license application (BLA) for the product in December 2022.
Other examples of biobetters include, Susvimo (based on Lucentis [ranibizumab]), Kadcyla (based on Herceptin [trastuzumab]), Gazva (based on Rituxan [rituximab]), and Neulasta Onpro (based on Neulasta [pegfilgrastim]).
Susvimo is an ocular implant that eliminates the need for intravitreal injections, like the ones required for administration of Lucentis. Kadcyla (ado-trastuzumab emtansine) is an antibody-drug conjugate that has been demonstrated to slow disease progression of HER2-positive advanced cancer compared with Herceptin. Gazyva (obinutuzumab) has a different method of action, is less immunogenic, and triggers greater cytotoxicity compared with Rituxan. Neulasta Onpro is an on-body injector version of Neulasta and currently is the leading pegfilgrastim product around the world.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
