The study cited is inadequately designed and precludes the drawing of conclusions regarding the comparative risk of FN in patients taking Amgen’s pegfilgrastim products depending on delivery method. It likewise does not support conclusions about any other FDA-licensed pegfilgrastim products.
- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
FDA Cautions Amgen About Pegfilgrastim Advertisement
An advertisement for the Onpro wearable pegfilgrastim injector kit has the potential to undermine sales of biosimilars, the FDA stated in a warning letter.
After receiving complaints through its Bad Ad Program, the FDA has issued a warning to Amgen to discontinue what it says are misleading claims about its Onpro pegfilgrastim wearable injector kit.
The FDA says a banner advertisement used by Amgen falsely claimed that the Onpro is more successful at reducing the incidence of febrile neutropenia (FN) than the prefilled syringe (PFS) form of administering pegfilgrastim.
The FDA said the study upon which Amgen based that claim was not geared to provide a reliable comparison of FN incidence between patient populations using Onpro vs PFS. The FDA also contends that the wording of the advertisement could create the false impression that biosimilar forms of pegfilgrastim, all of which are in PFS form, are inferior to Amgen’s wearable injector kit.
“These violations are concerning from a public health perspective because this promotional communication’s misleading claims could cause health care providers to conclude that Neulasta delivered via the Onpro on-body injector is more effective than Neulasta delivered via PFS or that it is more effective than FDA-licensed biosimilar pegfilgrastim products, which are only delivered via PFS,” the FDA wrote in an “untitled letter.”
In terms of severity, an untitled letter from the FDA is 1 step below a "warning letter," but still is intended to point out noncompliance with regulatory standards.
For its part, Amgen has indicated an intention to work with the FDA to resolve the issue. In a statement, it touted the demonstrated efficacy of Neulasta, but did not directly address the advertising message the FDA has objected to.
“There is a robust body of clinical and real-world evidence that demonstrates that when used every cycle, Neulasta significantly reduced the incidence of febrile neutropenia (FN) as well as FN-related events, including FN-related hospitalization and the use of anti-infective drugs in patients undergoing chemotherapy,” Amgen said in a statement to The Center for Biosimilars®.
The company noted it has a $2-billion stake in biosimilars, implying that running them down would not be to its benefit.
Amgen said the FDA's concerns relate to a banner ad directed at health care providers about a "real-world evidence" study for Neulasta used in both the Onpro and PFS study arms.
The FDA contends the ad did not make sufficiently clear that Neulasta, Amgen’s originator product, was also used in the PFS arm, and so merely using the nonproprietary name pegfilgrastim could tarnish biosimilars by association.
The FDA also takes issue with the following claims used in the advertisement:
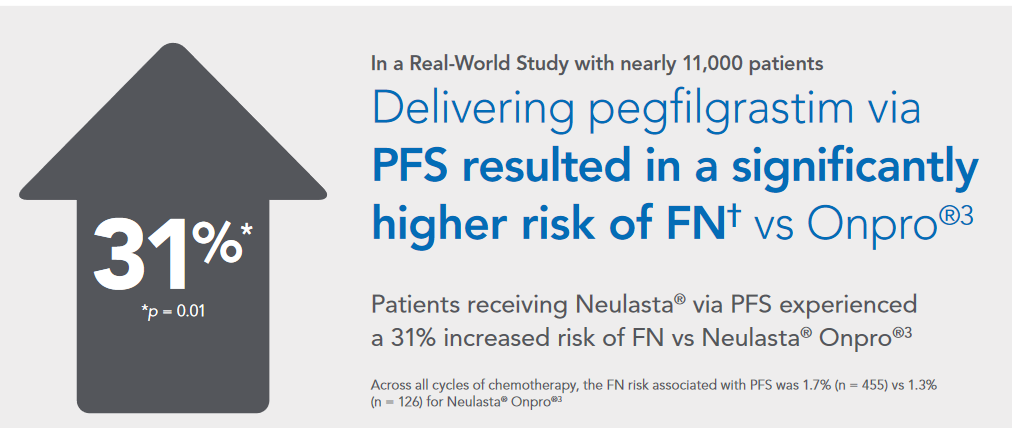
- “In a real world study with nearly 11,000 patients, pegfilgrastim PFS resulted in a significantly higher risk of FN vs Onpro.”
- "Across all cycles of chemotherapy, the incidence of FN associated with prefilled syringe (PFS) was 1.7% (n = 455) vs 1.3% (n = 126) for Neulasta Onpro.”
- “With PFS, FN incidence increased by 31% vs Onpro.”
The FDA contends that “multiple limitations” of the retrospective study used to justify these assertions makes such assertions very unsound.
The FDA also contends Amgen cannot fairly state that the alleged higher risk of FN with PFS reached the level of statistical significance. “Accordingly, reporting a P value for the claims and presentations in…the banner is misleading,” the agency wrote.
It said the algorithm chosen to select study participants relied on diagnosis codes for neutropenia and fever or infection and no information was provided to the FDA's Office of Prescription Drug Promotion about the sensitivity or positive predictive value of the algorithm or diagnostic codes.
“The use of an unvalidated algorithm with unknown performance characteristics is a significant limitation because of the potential for misclassification of patients at the onset of the study,” the FDA wrote.
The FDA also noted the purported 31% increase in incidence of FN was based on the difference between small proportions of patients who experienced FN in the PFS vs on-body injector groups (1.7% vs 1.3%, respectively). “It cannot be ruled out that selection bias is entirely responsible for the observed risk difference,” the FDA wrote.
Amgen was given 15 days to respond to the FDA with its reasoning and any supporting information to justify the advertising claims it has made.
The FDA noted the potential that Amgen had made similar claims in other advertisements and asked for corrections. The advertisment provided for story illustration can be found here.
In a statement, the Biosimilars Forum, which is a trade association of biosimilars companies, applauded the FDA's warning notice.
"Misinformation about the safety and efficacy of biosimilars emanating from market-leading brand-name drug companies is rampant," the group wrote. "The Biosimilars Forum has consistently engaged with the FDA and Federal Trade Commission (FTC) on this issue since day one, including calling on both agencies to take aggressive actions."
“Spreading false claims and misinformation harms patients and is a barrier to access to safe, effective and lower-cost biosimilars,” said Meaghan R. Smith, executive director of the Biosimilars Forum.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.