- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
FDA Issues Another CRL for Alvotech’s Adalimumab Biosimilar
In a complete response letter (CRL) for Alvotech, the FDA cited “deficiencies” in the company’s manufacturing facility during a reinspection, further delaying the approval for the ninth adalimumab biosimilar until potentially June 2023.
Alvotech's logo

The FDA issued a second complete response letter (CRL) to Alvotech for its adalimumab biosimilar (AVT02) after reinspecting the company’s Iceland-based manufacturing facility in March 2023.
The agency conveyed that the manufacturing still exhibited some “deficiencies,” but no issues with the biosimilar product itself, including data regarding safety or efficacy, were found. The biologics license application for AVT02 included information that demonstrated biosimilarity between the biosimilar and the reference product (Humira), as well as information from a switching study, in hopes of obtaining an interchangeability designation.
On April 3, Alvotech provided the FDA with responses to the agency’s inspection observations and is now waiting for a reply. Alvotech said that it will comply with the agency’s requests and that it still plans to pursue FDA approval. The FDA’s new decision date is set for June 28, 2023.
AVT02 has been approved in some international markets, including the European Union, where it was approved by the European Commission in December 2021 and is marketed as Hukyndra. It was also approved in Canada in January 2022, where is marked as Simlandi, and Australia in November 2022, where it’s marketed under 2 brand names: Ciptunec and Ardalicip.
In Canada, AVT02 is marketed under the name Simlandi. Pictured is a computerized image of the Simlandi auto-injector pen.

Adalimumab products are used to treat a number of inflammatory conditions, including:
- Rheumatoid arthritis in adults
- Polyarticular juvenile idiopathic arthritis
- Psoriatic arthritis
- Ankylosing spondylitis
- Crohn disease
- Ulcerative colitis
- Chronic plaque psoriasis
Alvotech’s planned launch date for AVT02 is in July 2023, when 8 other adalimumab biosimilars are planned to launch. Assuming AVT02 is approved in time for the company to stick to its launch timeline, AVT02 would join Amjevita (Amgen), the first adalimumab biosimilar to enter the market, and launch alongside Cyltezo (Boehringer Ingelheim), Hadlima (Organon/Samsung Bioepis), Hulio (Biocon Biologics), Yusimry (Coherus Biosciences), Abrilada (Pfizer), Hyrimoz (Sandoz), and Idacio (Fresenius Kabi).
Additionally, AVT02 is a citrate-free, high-concentration adalimumab biosimilar. The citrate-free element allows for less injection site pain for patients and the high-concentration formulation allows for a higher volume administration, meaning patients will require less doses. If approved with the interchangeability designation, it would be the only high-concentration adalimumab biosimilar to have one. It would also be the ninth adalimumab biosimilar overall and fifth overall to receive interchangeability.
FDA approved adalimumab biosimilars
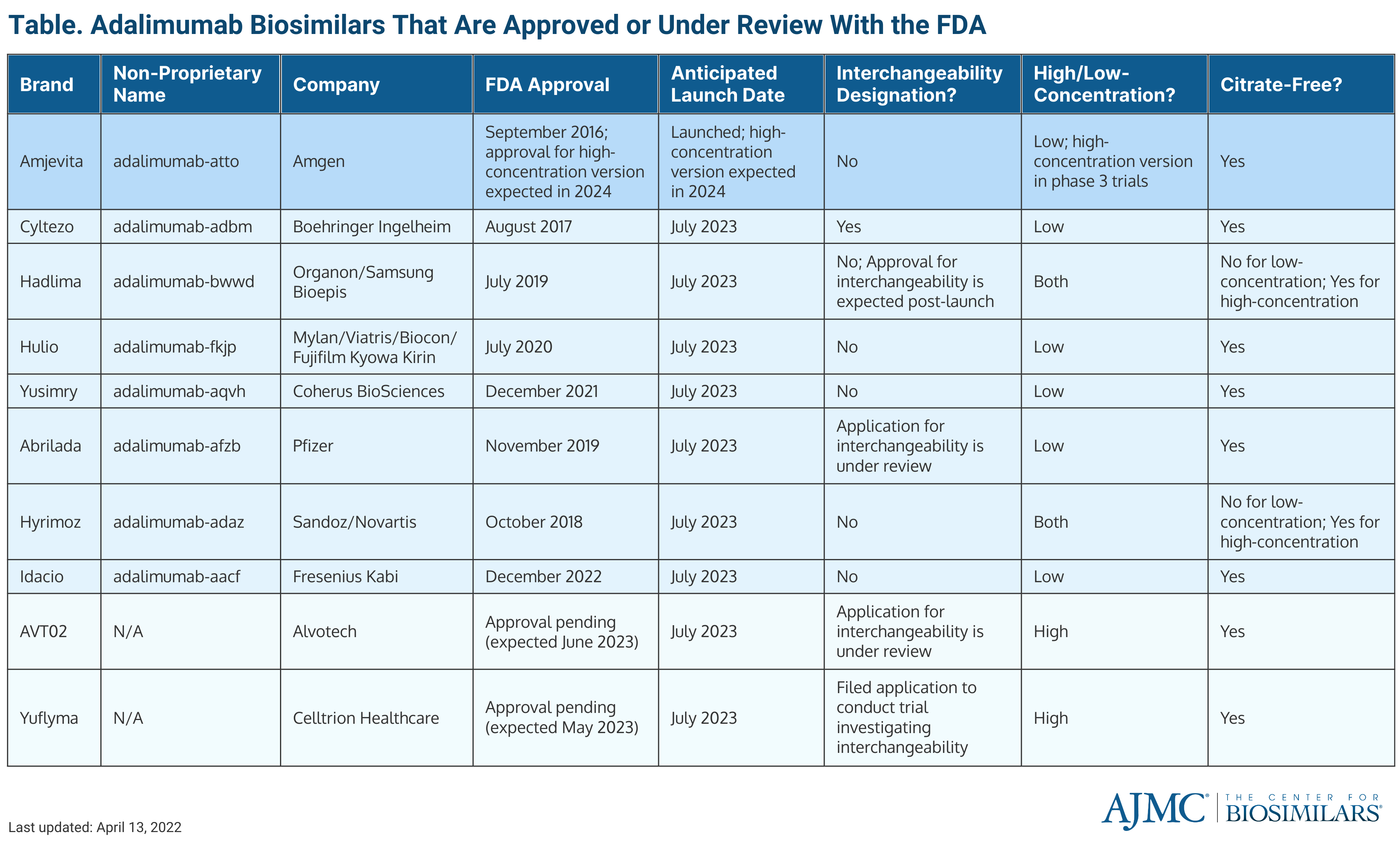
Celltrion Healthcare, whose high-concentration adalimumab biosimilar (Yuflyma) is expected to be approved in May 2023 and launch in July 2023, is also seeking interchangeability.
Interchangeability is a regulatory designation unique to the United States that allows for pharmacy benefit biosimilars to be exchanged for a reference product or another biosimilar without waiting for a physician to approve it, provided state laws allow it. It is intended to increase access and convenience for patients when obtaining their prescriptions.
“We look forward to working with the FDA to resolve any outstanding issues identified in the reinspection…. We are committed to manufacturing AVT02 for patients in the United States, especially a potentially differentiated Humira biosimilar that provides a high-concentration formulation and is interchangeable,” said Robert Wessman, chairman and CEO of Alvotech, in a statement.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
