Key Takeaways
- Although biosimilar competition has resulted in millions of dollars in savings for Medicare Part B plans and enrollees, opportunities to further reduce spending remain.
- Greater use of biosimilars could have saved Part B plans and enrollees $179 million, and the implementation of an least costly alternative (LCA) payment policy could have saved $419 million.
- HHS Office of Inspector General researchers suggest CMS monitor biosimilar trends and explore payment changes, potentially extending Center for Medicare and Medicaid Innovation models or seeking legislative authority, such as an LCA policy, to maximize savings for Part B and enrollees.
Biosimilar competition has resulted in lower drug costs for both Medicare Part B programs and beneficiaries; however, opportunities to further reduce spending through increased use of biosimilars or utilization of new payment policies remain, according to an HHS report.
The report, published by the Office of Inspector General (OIG), found that Part B and enrollee spending on biosimilars could have been reduced by $179 million in 2021 if more biosimilars had been used as frequently as the most-used biosimilars. Additionally, the implementation of alternative payment policies, such as a least costly alternative (LCA) policy, could have shrunk Part B and enrollee spending by $419 million, even without an increase in biosimilar use.
“To reduce Part B and enrollee spending on biologics, we recommend that [CMS] pursue one or more payment changes that could further realize savings from biosimilars for Part B and enrollees, which could include seeking additional legislative authority,” wrote the authors. "CMS did not explicitly concur or nonconcur with our recommendation, but stated that it is committed to taking action, as appropriate, within its authority."
The OIG study analyzed trends in pricing and use rates of biosimilars and their reference agents in the fee-for-service Medicare Part B programs from 2015 to 2021. The researchers estimated how Part B and enrollee spending in 2021 could have changed with increased use of biosimilars and an LCA payment policy. The review only included biosimilars available on the US market and covered by Part B during the analysis period.
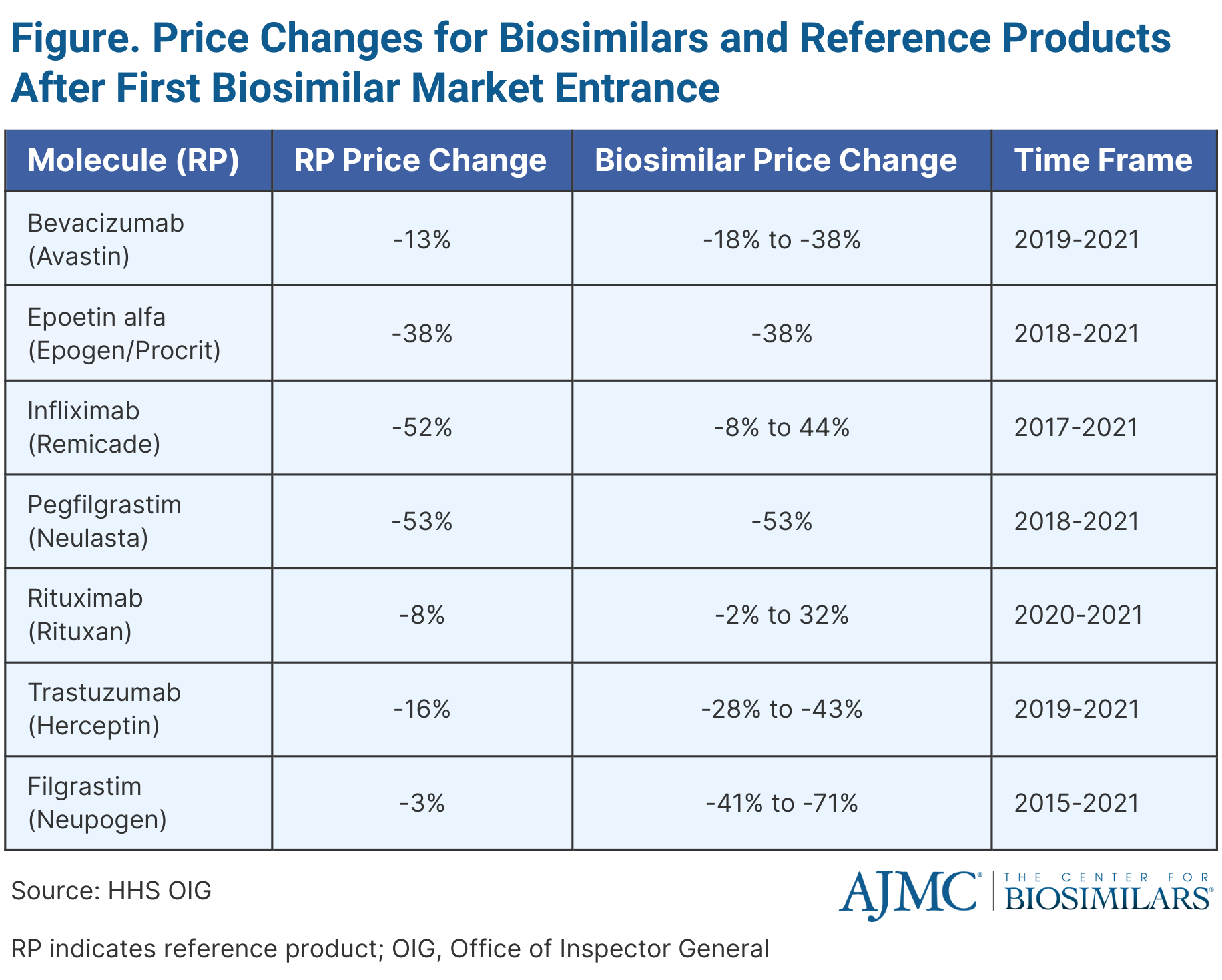
Results showed that prices for select biosimilars and originators (bevacizumab, epoetin alfa, infliximab, pegfilgrastim, rituximab, trastuzumab, filgrastim) fell once biosimilar alternatives entered the market. (Figure)
The trend of biosimilars having lower prices than their reference products generally continued, and although biosimilar prices remained lower overall, some reference product prices decreased significantly, making them cheaper than certain biosimilars by the end of 2021. The decreases helped lower program and enrollee costs, with an illustrative example showing potential additional spending of $264 million on a specific drug if reference product prices had not decreased after the introduction of biosimilar competitors.
Overall utilization of biosimilars for the aforementioned molecules increased, ranging from 23% (infliximab) to 82% (filgrastim). While some biosimilars, such as filgrastim biosimilars, are approaching or surpassing the use rates of their reference products, pegfilgrastim and infliximab biosimilars exhibited lower initial use rates—below 16% a year after introduction—and maintained much lower use rates compared to other biosimilars by the end of 2021.
Increased use of more affordable biosimilars, particularly those costing less than their reference products, could have resulted in a reduction of $179 million in Part B and enrollee spending in 2021, representing 4% of the $4.4 billion spent on these biosimilars and reference products as well as $1219 in annual savings per enrollee.
Implementation of an LCA policy could have reduced Part B and enrollee spending on biologics in 2021 by $419 million, about 9% of the total spending on biosimilars and reference products. The researchers recommended that policymakers weigh the potential spending reduction of an LCA policy against concerns about its broader effects, including worries that it may lead providers to prioritize affordability over appropriateness in treatment decisions, impact on providers' comfort with biosimilars, and potential disincentives for future biosimilar development due to short-term price competition.
Regarding study limitations, the estimates of changes in Part B spending and enrollee spending related to increased biosimilar use or an LCA policy in 2021 were not precise, as they did not consider factors like the impact on average sales prices, achievability of increased use rates, dynamic effects on manufacturer prices or provider incentives, and other factors such as prescriber preferences or the effects of sequestration on Medicare payment amounts.
CMS commented on the report noting how the Inflation Reduction Act will impact biosimilar use and spending, highlighting the temporary Part B increase in reimbursement payments for certain biosimilars that went into effect after the study period concluded.
“It is also important to note that factors outside of coverage and payment policy may affect provider and beneficiary preferences for a reference product versus the biosimilars.... CMS will continue to research and pursue strategies within our statutory authority to address both cost and market competition concerns.”
Reference
HHS OIG. Biosimilars have lowered costs for Medicare Part B and enrollees, but opportunities for substantial spending reductions still exist. HHS website. Published September 2023. Accessed November 27, 2023.