- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
How State Substitution Laws Shape Insulin Biosimilar Adoption
States with fewer restrictions on biosimilar substitution tend to see higher uptake of interchangeable insulin glargine, showing how even small policy details can significantly influence biosimilar adoption and expand access to more affordable insulin.
States with less restrictive biosimilar substitution laws were more likely to have higher uptake of an interchangeable insulin glargine biosimilar, underscoring the impact state regulations can have on biosimilar adoption, according to a recent study published in JAMA.1
This study was conducted to explore whether state-level substitution laws impact the uptake of interchangeable biosimilars in the US. When biosimilars first entered the market, many clinicians were concerned about the safety and efficacy of these lower-cost biologics, particularly the safety of switching from the originator to an alternative with “no clinically meaningful differences” that isn’t a generic.2
All US states have laws regulating how interchangeability works, with 4 states and Puerto Rico passing laws that restrict interchangeability entirely. Most state laws pertain to whether patients or providers need to be notified that a substitution has occurred, potentially affecting biosimilar use.
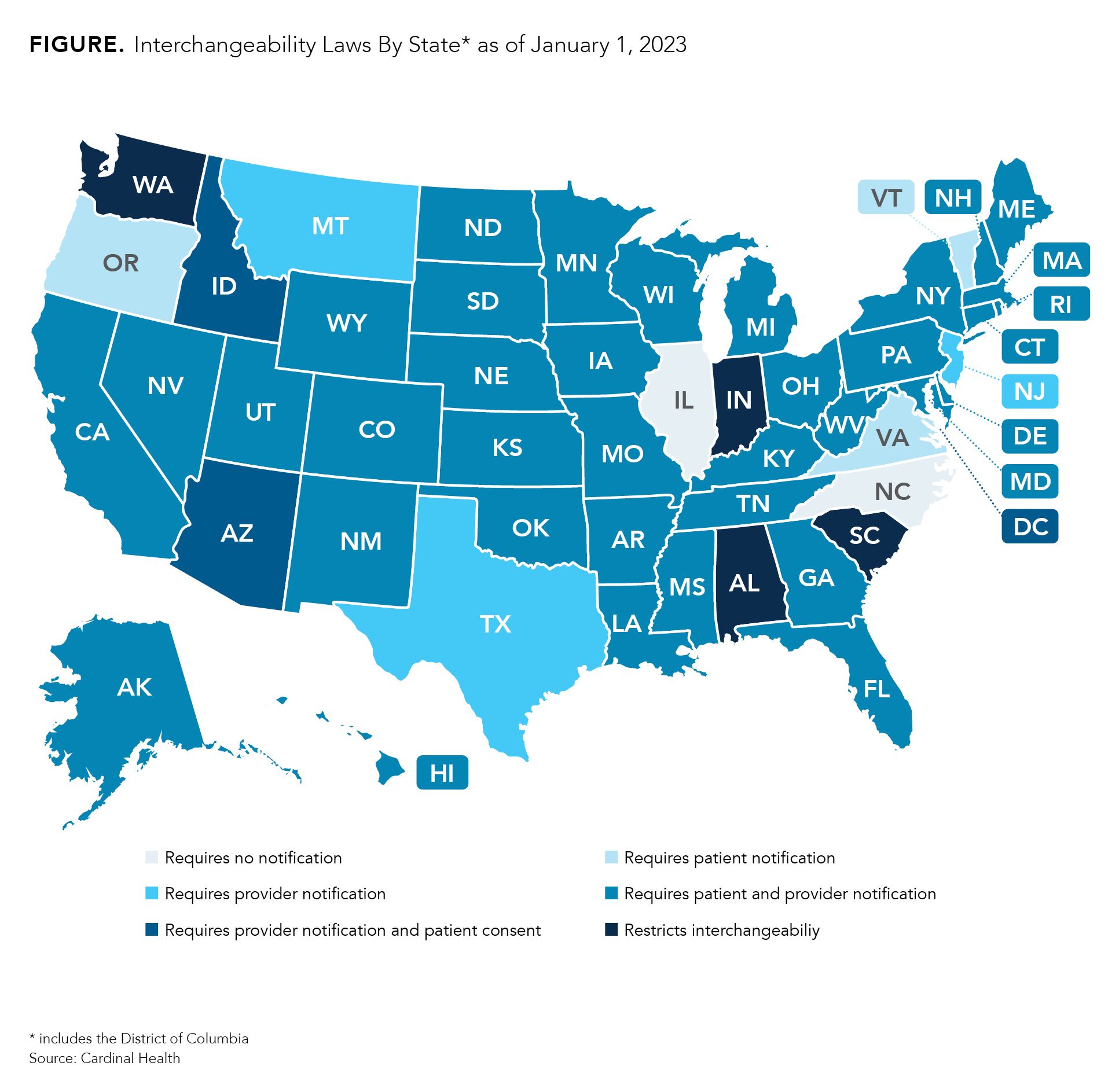
To address this, the US created an interchangeability designation allowing pharmacists to substitute certain biosimilars without prescriber approval.3 Although the FDA requires biosimilar manufacturers to submit switching study data to obtain the label, insulin biosimilars are exempt from this due to a 2020 rule based on a century's worth of data showing the safety of switching between equivalent insulin products.4
However, every US state has laws regulating how interchangeability works, with 4 states and Puerto Rico passing laws that restrict interchangeability entirely.5 Most state laws pertain to whether patients or providers need to be notified that a substitution has occurred, potentially affecting biosimilar use. Because no previous research has investigated this issue, the present study aimed to assess how these substitution laws influence the dispensing of insulin glargine-yfgn (Semglee; Mylan Pharmaceuticals), the first biosimilar to be deemed interchangeable.1,6
Researchers conducted a retrospective analysis using MarketScan pharmacy claims from November 2020 to November 2022 to examine how state biosimilar substitution laws affected uptake of interchangeable insulin glargine-yfgn. They used a 7-point scale to categorize the restrictiveness of the laws and compared changes in the use of insulin glargine-yfgn before and after its launch in November 2021. A difference-in-differences analysis measured the impact, adjusting for enrollee characteristics and time trends. Several sensitivity checks ensured results weren’t influenced by other insulin products or outlier states.
The study included 487,281 insulin glargine prescription fills (mean age, 49.5 years; male, 56.9%), with 32.5% from states with less restrictive substitution laws and 67.6% from more restrictive states. After the launch of insulin glargine-yfgn, market share increased by 7.03 percentage points (95% CI, 1.89-12.18; P = .008) in less restrictive states compared with more restrictive ones, while the market share of the reference insulin declined by 6.48 percentage points (95% CI, −11.70 to −1.26; P = .02).
No significant changes were observed for the noninterchangeable biosimilar (−0.24 percentage points; P = .68). Specific restrictive laws—like enhanced physician notification (−8.15 percentage points; P < .001) and refill notifications (−4.68 percentage points; P = .03)—were most associated with lower biosimilar uptake. Results remained consistent across subsequent sensitivity analyses.
The authors recommended some solutions. “One fruitful area of reform is revisiting the requirements regarding physician and patient notifications, given their ubiquity and pronounced associations with substitution. For example, waiving notification for refills or allowing a longer time frame for notification may lower the administrative burden for the substituting pharmacist,” they explained.
However, greater substitution may not guarantee savings for patients, as insurance plans may still favor reference products, which carry higher rebates than biosimilars.7 The study also had some limitations, including potential unmeasured factors, like prescribing habits or patient preferences, as well as a lack of plan-level data and possible misclassification of state laws.1
Still, the authors emphasized that increased biosimilar use could enhance price competition, potentially lowering net drug prices over time. “These dynamics, along with other barriers to market entry, need to be more thoroughly explored as the biosimilar markets mature.”
References
1. Kwon Y, Sarpatwari A, Dusetzina SB. State substitution laws and uptake of an interchangeable insulin biosimilar. JAMA Health Forum. 2025;6(4):e250406. doi:10.1001/jamahealthforum.2025.0406
2. Biological products definitions. FDA. October 12, 2017. Updated October 16, 2017. Accessed April 14, 2025. https://www.fda.gov/files/drugs/published/Biological-Product-Definitions.pdf
3. Interchangeable biological products. FDA. July 21, 2021. Updated July 27, 2021. Accessed April 14, 2025. https://www.fda.gov/media/151094/download
4. Hagen T. It's a new set of rules for insulin products under 351(k). The Center for Biosimilars®. June 15, 2020. Accessed April 15, 2024. https://www.centerforbiosimilars.com/view/its-a-new-set-of-rules-for-insulin-products-under-351k
5. State laws for biosimilar interchangeability. Cardinal Health. https://www.cardinalhealth.com/en/product-solutions/pharmaceutical-products/biosimilars/state-regulations-for-biosimilar.html
6. Hagen T. FDA approves Semglee insulin glargine as the first interchangeable biosimilar. The Center for Biosimilars. July 28, 2021. Accessed April 14, 2025. https://www.centerforbiosimilars.com/view/fda-approves-semglee-insulin-glargine-as-first-interchangeable-biosimilar
7. Mouslim MC, Rashidi ES, Levy JF, Socal MP, Trujillo AJ. The price paradox of biosimilar-like long-acting insulin. Am J Manag Care. 2022;28(11):e405-e410. doi:10.37765/ajmc.2022.89265
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
