- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Investigators Measure Pegfilgrastim Biosimilar Savings Potential
The savings from pegfilgrastim biosimilars rapidly add up, according to a study simulation that accounted for hospital costs associated with the on-body injector.
Whether or not estimated failure rates of the pegfilgrastim onbody injector (OBI; Onpro, Neulasta) are factored in, pegfilgrastim biosimilars have the potential to generate significant savings, investigators found in a model simulation analysis. Their study evaluated the potential savings of the pegfilgrastim biosimilar Fulphila used as prophylaxis treatment against chemotherapy-induced neutropenia (CIN).
The OBI, an Amgen follow-on product to Neulasta, is designed to automatically deliver a pegfilgrastim injection outside the clinic, but failure rates for this device have ranged from 1% to 7%, according to authors of the study. Use of the OBI has surged during the COVID-19 pandemic because of the convenience of at-home injections.
The study model simulated a panel of 15,000 US patients at risk for CIN or febrile neutropenia (FN) and estimated the savings from converting patients from pegfilgrastim with OBI to the pegfilgrastim biosimilar. The savings ranged from $481,259 for converting 10% of patients for 1 cycle of prophylaxis to $29 million for converting 100% of patients for 6 cycles.
When authors of the study adjusted for potential pegfilgrastim-OBI device failure and associated FN-related hospitalization costs, the estimated savings for 6 cycles ranged from $2.9 million for 10% conversion and a 1% failure rate to $32.2 million for 100% conversion and a 7% failure rate.
The authors also quantified the expanded access to pegfilgrastim prophylaxis or antineoplastic treatments that would be possible with these savings. Based on their analysis of 100% conversion to the biosimilar and a 7% failure rate, up to 11% more patients could receive a 6-cycle regimen of prophylaxis with the biosimilar, or 5% more patients could receive antineoplastic treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
For patients undergoing myelosuppressive chemotherapy, CIN and FN are “potentially life-threatening complications,” the authors said, and treatment with granulocyte colony-stimulating factor (G-CSF), which stimulates production, maturation, and survival of neutrophils, is used to reduce the risk of these conditions in many patients. Pegfilgrastim is a pegylated G-CSF, a long-acting form of filgrastim, available as originator pegfilgrastim, OBI, or biosimilar.
The authors noted that pegfilgrastim treatment for CIN/FN prophylaxis is recommended 24 to 72 hours following chemotherapy, which presents a logistical challenge, because patients must return to the oncology clinic the day after their treatment. Real-world data suggest that same-day administration “is becoming increasingly common.” In 2015, the FDA approved the Amgen OBI device for pegfilgrastim administration, which is attached to the patient on the same day as chemotherapy treatment and is designed to automatically deliver a pegfilgrastim injection the next day, approximately 27 hours later.
The authors explained that because the device administers a single dose of pegfilgrastim, “a missed or partial dose resulting from device failure may significantly increase the risk for FN and FN-related hospitalizations.”
In the study, cost savings were calculated first without and then with hypothetical OBI device failure and associated FN-related hospitalization costs. The medication costs were based on average sales prices from 2020, and costs of administering the medications were included. Costs were calculated from the payer’s perspective; patient costs such as co-pays were excluded. FN-related hospitalization rates and costs were based on existing data for US patients with non-Hodgkin lymphoma.
Cost Savings in 15,000 Hypothetical US Patients
The authors estimated that per-patient treatment with pegfilgrastim biosimilar rather than pegfilgrastim-OBI would save $321 for 1 cycle and $1925 for 6 cycles (Table 1). Across the panel of 15,000 patients, the model calculated potential cost savings ranging from $481,259 if 10% of patients converted to the biosimilar in 1 treatment cycle to $28.9 million for 100% of patients converted over 6 cycles.
Click to enlarge
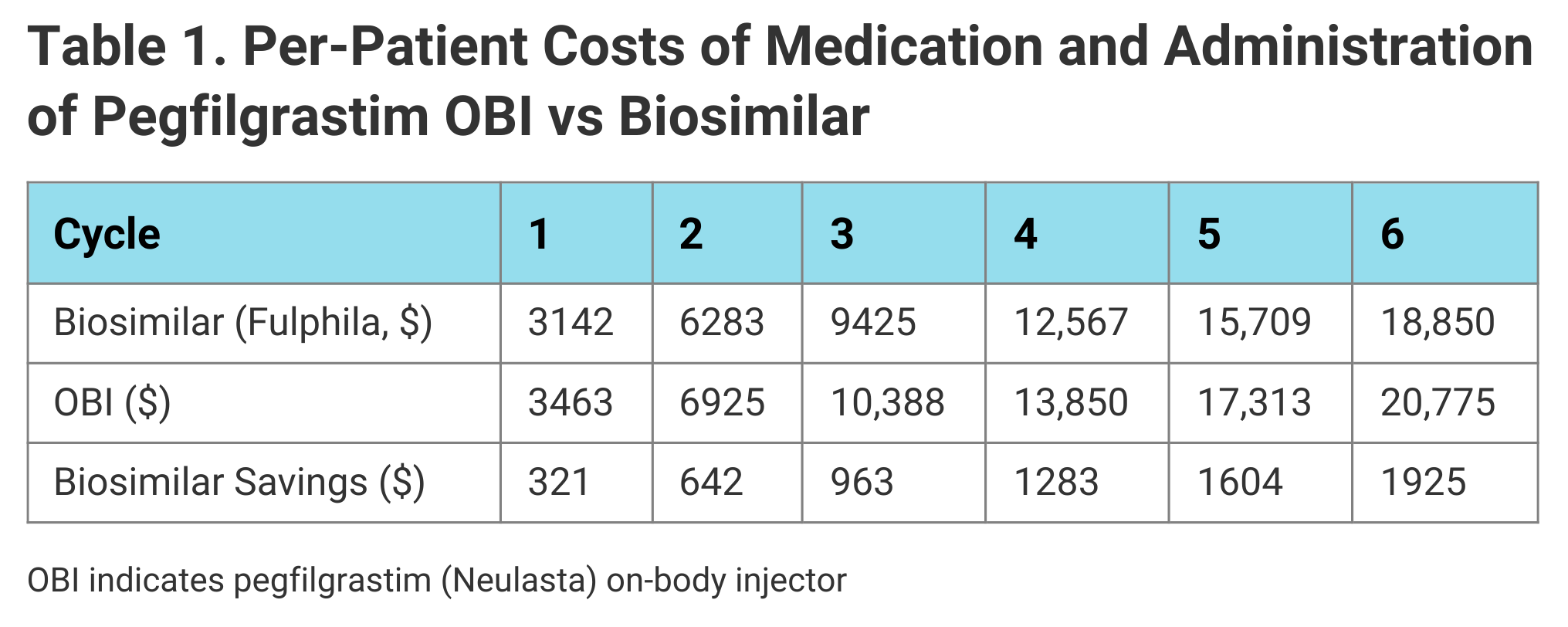
By reallocating the savings over 6 cycles, if 10% of patients received pegfilgrastim biosimilar instead of pegfilgrastim-OBI, 919 additional doses of pegfilgrastim biosimilar could be purchased; if 100% of patients received pegfilgrastim biosimilar, 9191 additional doses could be purchased, allowing an additional 1532 patients to be treated with the biosimilar. Alternatively, if cost savings were reallocated to purchase additional doses of anticancer therapy with R-CHOP, at a 100% conversion rate for 6 cycles, 744 additional patients could be treated.
The number of patients needed to convert (NNC) from pegfilgrastim-OBI to pegfilgrastim biosimilar to purchase 1 additional dose of the biosimilar was 9.79 patients for 1 cycle and 1.63 for 6 cycles. To purchase 1 additional dose of R-CHOP, the NNC was 20.17 patients for 1 cycle and 3.36 patients for 6 cycles.
Cost Savings Adjusted for OBI Failure
Incorporating OBI device failure rates of 1% to 7% and the associated FN-related hospitalization costs into their simulation, the authors projected that over 6 cycles of prophylaxis, cost savings in 15,000 hypothetical patients would range from $2.9 million for a 10% rate of conversion to the biosimilar and device failure rate of 1% to $32.2 million for a 100% conversion rate and 7% failure rate. FN-related hospitalization costs based on a 7% OBI failure rate over 6 cycles would add up to $3.4 million (Table 2).
Click to enlarge
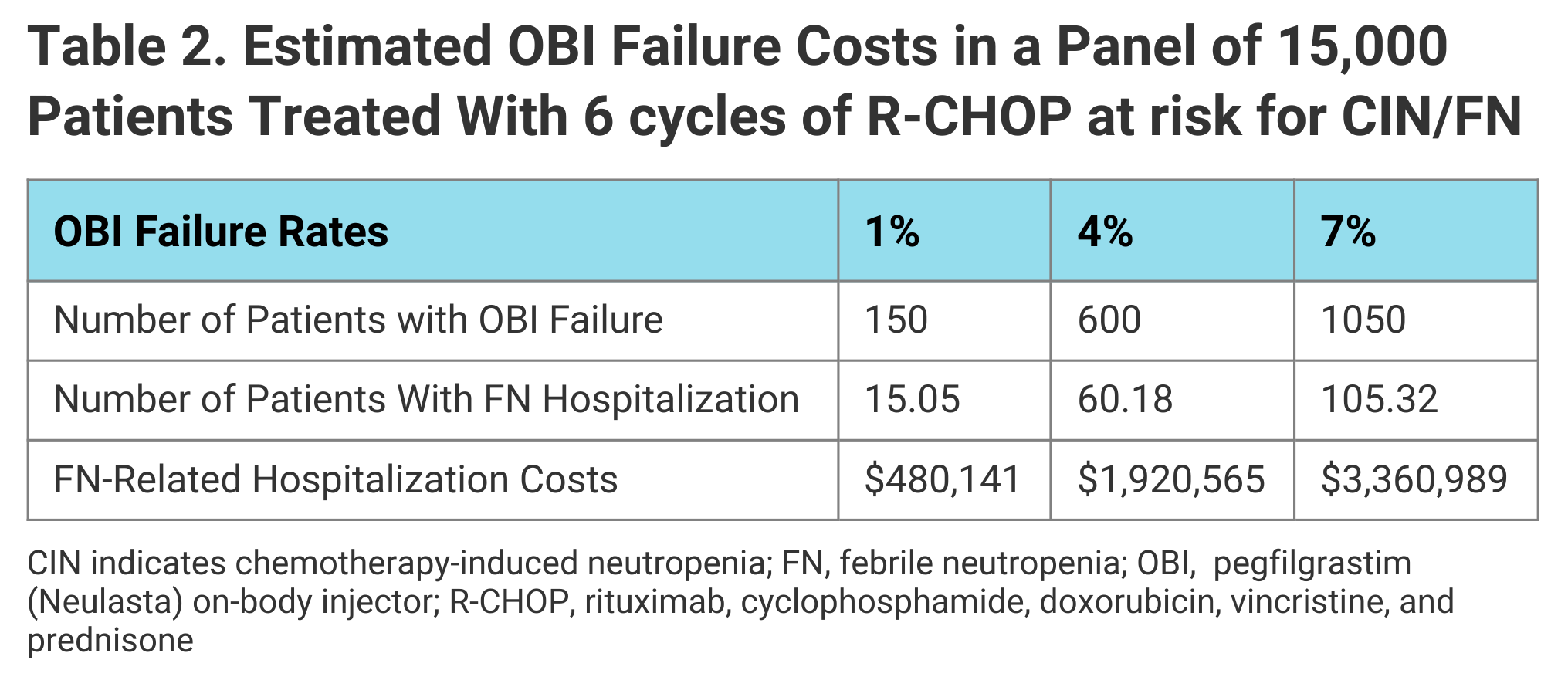
The model’s results suggested reallocating those savings could provide up to 10,261 additional doses of the biosimilar or 4982 additional doses of R-CHOP.
The authors concluded that “biosimilar pegfilgrastim is a clinically relevant, cost-efficient, and safe alternative to reference pegfilgrastim-OBI for CIN/FN prophylaxis,” that the cost savings “increase markedly when also accounting for pegfilgrastim-OBI failure and associated FN-related hospitalizations,” and these cost savings could allow “significant numbers” of additional patients to access prophylaxis with the biosimilar or antineoplastic therapy with R-CHOP.
Reference
McBride A, MacDonald K, Fuentes-Alburo A, Abraham I. Conversion from pegfilgrastim with on-body injector to pegfilgrastim-jmdb: cost-efficiency analysis and budget-neutral expanded access to prophylaxis and treatment. J Med Econ. Published online April 18, 2021. doi:10.1080/13696998.2021.1916863
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.