- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Investigators Test Reliability of Interchangeability Switching Studies
How many switches between a biosimilar and reference product will be sufficient to demonstrate that the risk of transitioning is minimal? A virtual study verifies the strength of FDA guidance.
Recently, biosimilar developers have sought to obtain “interchangeable” status for their products so that pharmacists can dispense them without first consulting the prescribing physicians.
But demonstrating that biosimilars are interchangeable with reference products (RPs) requires that developers perform studies that ensure that the same clinical outcomes are achieved whether patients switch back and forth between RP and biosimilar multiple times or remain on RP throughout.
Based on a recent simulation study, investigators said the optimal number of switches that must be performed is 3 to reliably conclude that outcomes would be the same. This means patients would start out on RP, switch to the biosimilar, switch back to RP, then switch back to the biosimilar again.
“At least 3 switches appeared necessary to optimize the detection of potential [pharmacokinetic] PK differences,” investigators wrote. They also evaluated the merits of a single switch and 5 switches, concluding 1 was inadequate and 5 would be more than necessary.
Their findings conform to actual FDA guidance on switching, which calls for 2 periods of patient exposure to both the biosimilar and RP (Figure).
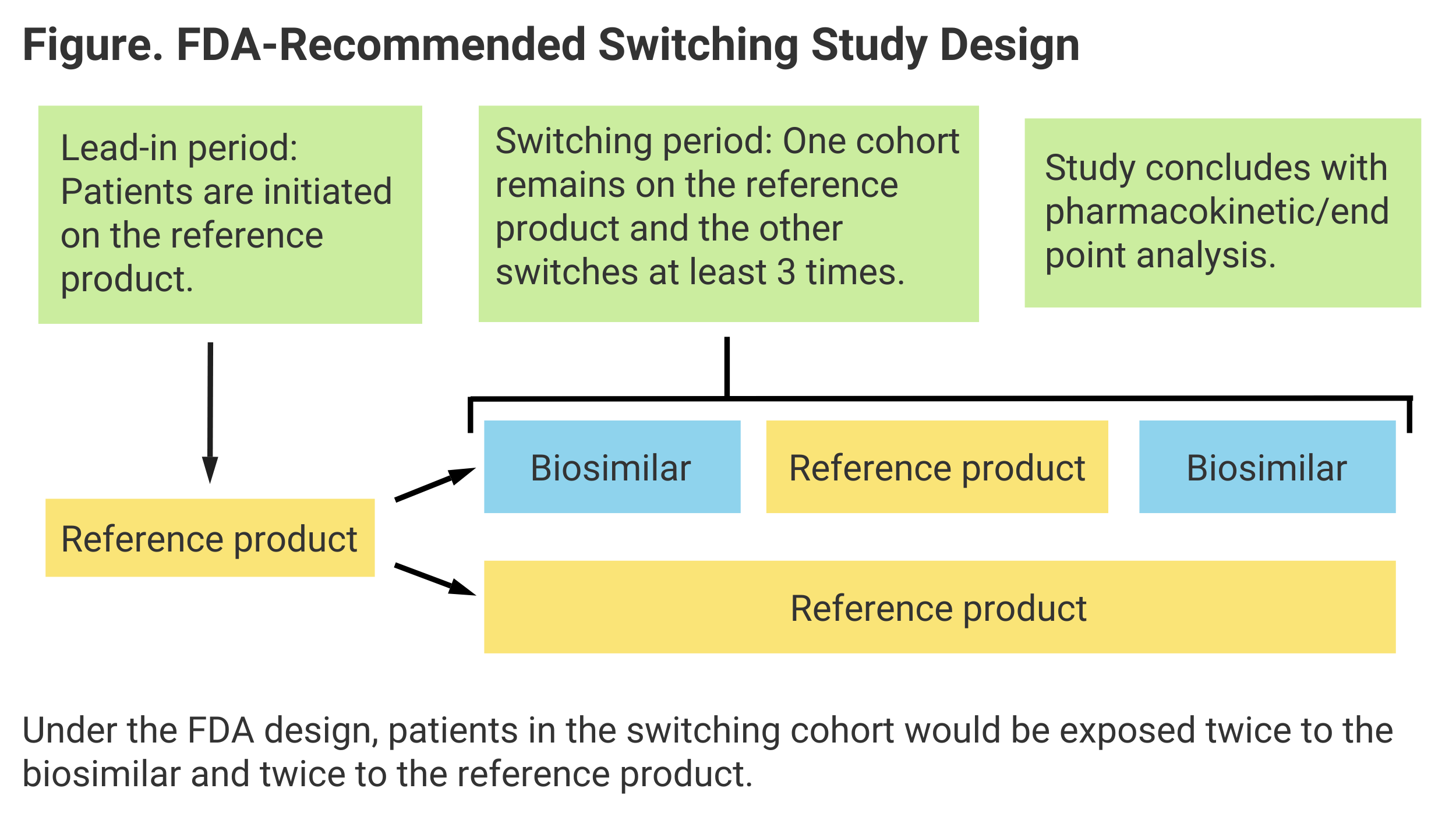
Investigators also concluded that the development of antidrug antibodies (ADAs) is significant in determining clinical equivalence of switching vs nonswitching, as is “area under the curve” (AUC), or extent of exposure to a drug and its clearance from the body.
“Our simulation work illustrates that a range of factors should be carefully considered when designing a switching study for the assessment of interchangeability between 2 biological products,” authors of the study wrote.
The difference between a biosimilar and an interchangeable can be confusing. For biosimilar approval, a manufacturer must demonstrate that its product has no clinically meaningful safety or efficacy differences from RP. For an interchangeable designation, a manufacturer must show that the product “can be expected to produce the same clinical result as the [RP] in any given patient,” and for this a switching study is necessary.
The first interchangeable biosimilar approved in the United States was an insulin glargine (Semglee; Mylan and Biocon Biologics), for which a switching study (INSTRIDE 3) that included the 3 recommended switches was performed. Interchangeability was granted July 2021.
Boehringer Ingelheim and Alvotech have each sought interchangeable status for proposed adalimumab biosimilars and have conducted switching studies. FDA decisions are pending.
Marketing Advantage
There is an inherent marketing advantage in an interchangeable designation. There is also the potential for health care savings as more of these products are approved, authors of the study on optimal switching said.
“The continued rise of biosimilar product development programs and regulatory approvals in the past few years has offered a solution to address the high costs of these new biologic treatments, and the lower costs of biosimilars are easing an ever-increasing strain on national health care systems. Interchangeable products have the potential to further lower health care costs and improve efficiency in the prescription dispensing process,” they wrote.
The purpose of a switching study is to demonstrate that the risk of switching to a biosimilar or back again doesn’t increase the risk of diminished safety or efficacy vs remaining on the originator product. To establish this, an analysis of PK and pharmacodynamic (PD) changes is recommended by the FDA.
“Changes in PK and/or PD concentrations or exposures may be the result of ADA formation,” the authors wrote. In their virtual study, they randomized patients (N = 200) 1:1 to the switching or nonswitching arms, and 750 mg of the reference or test product were hypothetically administered each cycle (4 weeks or 28 days).
Investigators evaluated ADA incidence during the lead-in (baseline) period with the RP treatment and the additional ADA incidence resulting from switching between periods. The end points were AUC and maximum concentration (Cmax) values from PK sampling. Cmax is the highest concentration of a drug in the bloodstream following dosing. Investigators concluded “Cmax was less sensitive for assessing the impact of ADA on PK.”
Reference
Ji P, ren Y, Li L, et al. Impact of switching on pharmacokinetics of therapeutic biologics and interchangeability assessment—a simulation study. J Clin Pharmacol. Published online August 19, 2021. doi:10.1111/1954
For a column on the significance of interchangeability by Jeff Baldetti, MBA, of Cardinal Health, click here.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.

