- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Multiple Switches Between Adalimumab Biosimilar GP2017 and Humira for Psoriasis Maintain Biosimilarity
Patients with moderate to severe plaque psoriasis who switched between the adalimumab biosimilar GP2017 and reference adalimumab did not impact immunogenicity, safety, or efficacy following up to 4 switches between both drugs.
Patients with moderate to severe plaque psoriasis who switched between the adalimumab biosimilar GP2017 and reference adalimumab did not impact immunogenicity, safety, or efficacy following up to 4 switches between both drugs. | Image Credit: pimentos - stock.adobe.com
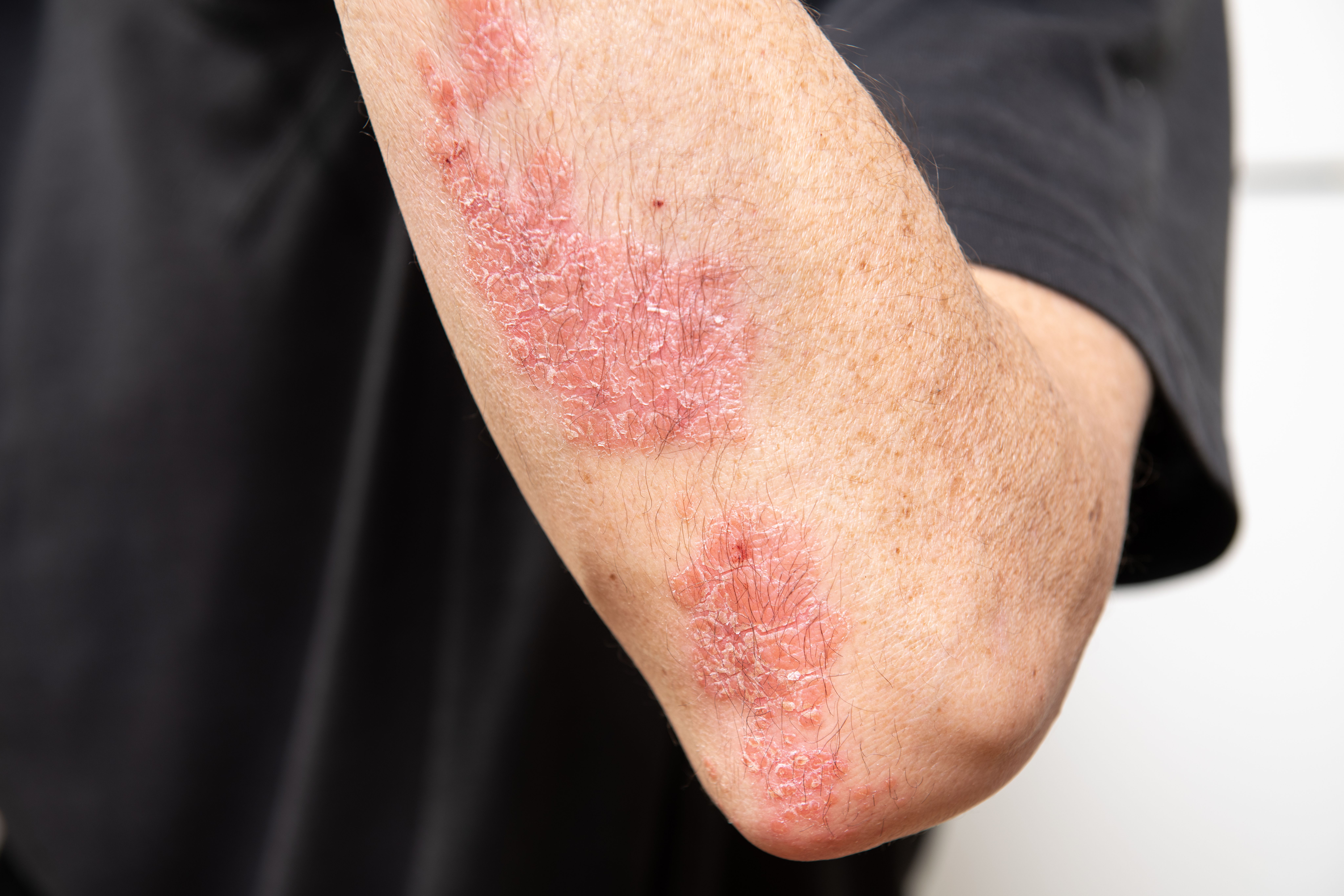
The adalimumab biosimilar GP2017 and reference adalimumab used to treat patients with moderate to severe plaque psoriasis did not significantly impact immunogenicity, safety, or efficacy, even after multiple switches, suggesting potential cost savings and improved access to biosimilars, according to a study published in Expert Opinion on Biological Therapy.1
A standard approval process confirms that biosimilars have the same efficacy, safety, and immunogenicity profile as the reference biologic. In the US, interchangeability is a legal distinction that established the biosimilar pathway, but it is not well understood. Health care professionals and other stakeholders often hold misconceptions that believe interchangeable biosimilars are better than biosimilars without interchangeability designation. However, there is an ongoing discussion between clinicians about the necessity of an interchangeability designation in the US, with many believing the additional demand of efficacy and safety data goes beyond the required evidence for approval.2
Last year, the FDA submitted draft guidance that argued biosimilars should receive interchangeability status without extensive switching studies, which would essentially remove interchangeability while maintaining status and complications.3,4 The Biologics Price Competition and Innovation Act may remove interchangeability in 2025, leaving an allowance for carrying over exclusivity to products with this status, speculated Sarfaraz K. Niazi, PhD.4
Researchers designed the ADACCESS trial (NCT02016105) to assess biosimilarity between the biosimilar adalimumab-adaz (Sandoz; GP2017) and reference adalimumab (AbbVie; Humira) in patients with moderate to severe chronic plaque psoriasis.1 The ADACCESS study was a 51-week multicenter, randomized, double-blind controlled confirmatory efficacy and safety study. Researchers continuously treated patients with reference adalimumab (cH group) and had patients who experienced 4 switches between the reference drug and GP2017 (H2H group).
Using electrochemiluminescence (ECL) antidrug antibody (ADA) assays, the ADA signal-to-noise (S/N) ratio, a continuous variable, has been proposed as an alternative approach for assessing the magnitude of ADA response. Researchers define ADA S/N as the ratio of the ECL signal of the sample divided by the negative control signal. The study authors investigated potential differences in immunogenicity based on ADA S/N ratio as an alternative end point.
There were 63 participants in the cH group and 127 participants in the H2H group who were considered for the ADA analysis. At week 17, the cH group had 58 participants, and the H2H group had 122 participants. By week 41, or 6 weeks after the fourth switch, there were 46 participants in the cH group and 100 participants in the H2H group. Both week 17 and week 41 were all within the defined margin of –0.16 to 0.16. The 90% CIs remained within the margin for all other time points.
The efficacy was comparable between the cH arm and H2H switching arm, showing that switching does not impact efficacy. Adverse events (AEs) and serious AEs were comparable and similar in terms of System Organ Class and Preferred Term levels. There were no patterns or imbalances between treatment groups regarding AEs with a suspected causal relationship to the study medication, or other safety information relevant to the benefit-risk assessment.
The ADA S/N ratio limits the study because it is more sensitive to showing differences in immunogenicity than a pharmacokinetic end point following single-dose administration of GP2017 in healthy patients. The hypothesis neglects data-driven confirmation, and the study does not have a positive control. Clinical studies and real-world settings have not observed increased immunogenicity upon reference product to biosimilar switching or upon repeated biosimilar switches.
“This could lead to decreased costs for development of biosimilars, which may result in savings for healthcare systems and improved access for patients to these drugs,” study authors concluded.
References
- LemkeL, BlauveltA, BrückmannI, et al. Comparing anti-drug antibody signal-to-noise ratios to assess immunogenicity and interchangeability in adalimumab biosimilar studies. Expert Opin Biol Ther. 24(12);1375-1385. doi:10.1080/14712598.2024.2428299
- Humphreys S. Understanding interchangeable biosimilars at the federal and state levels. The American Journal of Managed Care®. 2023;29(7):545-548. doi:10.37765/ajmc.2023.89419
- Jeremias S. FDA draft guidance removes switching study requirements for biosimilar interchangeability. The Center for Biosimilars®. June 20, 2024. Accessed March 20, 2025. https://www.centerforbiosimilars.com/view/fda-draft-guidance-removes-switching-study-requirements-for-biosimilar-interchangeability
- Niazi SK. BioRationality– the evolution of the BPCIA and the bright future of biosimilars in the US. The Center for Biosimilars®. October 7, 2024. Accessed March 19, 2025. https://www.centerforbiosimilars.com/view/biorationality-the-evolution-of-the-bpcia-and-the-bright-future-of-biosimilars-in-the-us
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
