- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
NCCN Issues Guidelines for Biosimilar Use
The National Comprehensive Cancer Network (NCCN) recommended providers contact their legislators to counteract single-biosimilar payer policies.
A new set of guidelines for the use of biosimilars from the National Comprehensive Cancer Network (NCCN) addresses challenges of stocking biosimilars, dealing with procurement errors, and obtaining payer authorizations.
It also advises health care providers to lobby for more liberal payer policies, stating that current payer preferences threaten the long-term existence of biosimilars. “We strongly recommend against single-source mandates of biosimilar products by insurance companies for a variety of patient safety and operational reasons,” the NCCN wrote.
Human error during the biosimilar procurement process is another challenge for practices, and the NCCN paper identified numerous other potential breakdowns and their effects on clinical efficiency (Table).
Click to enlarge
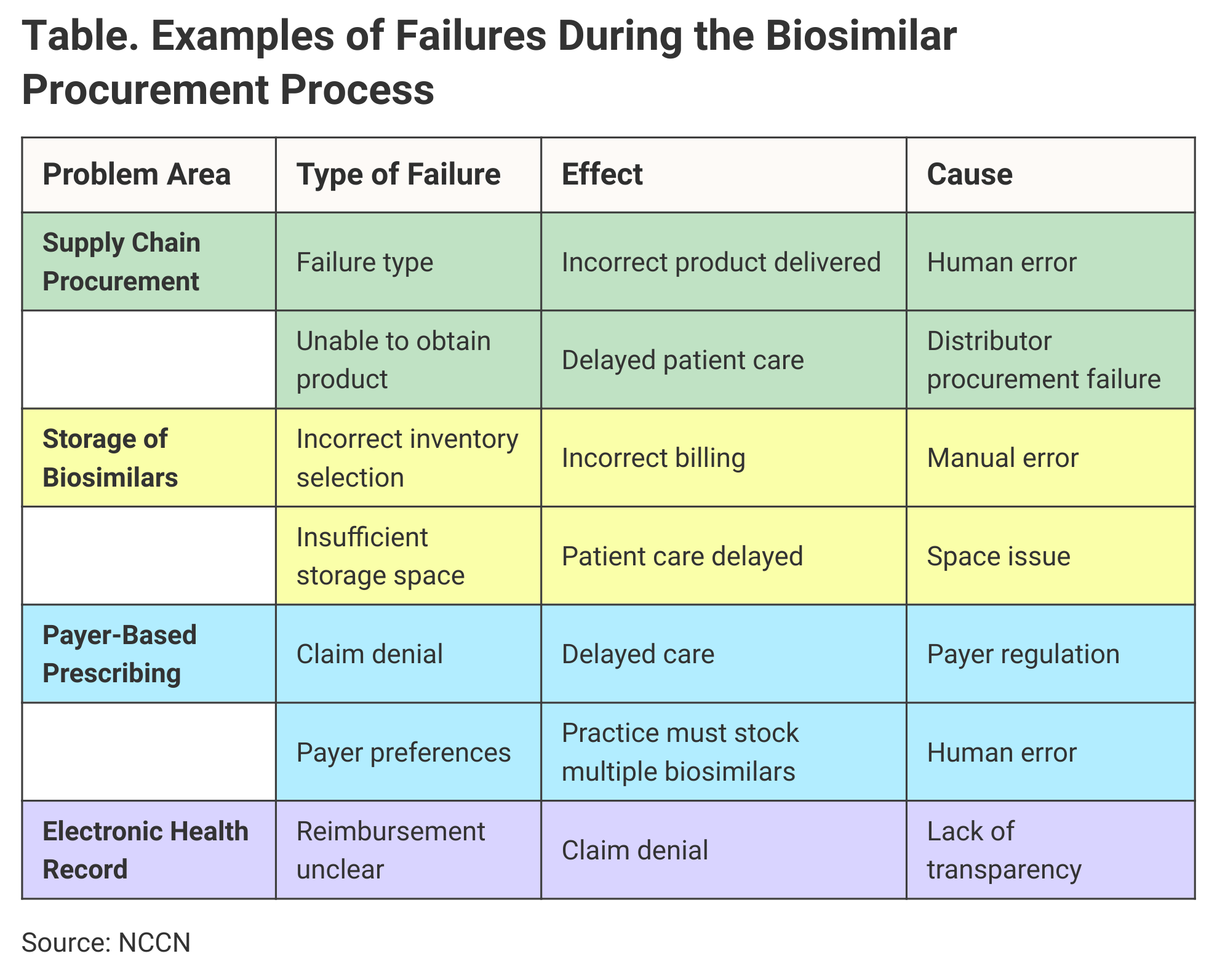
The NCCN said its recommendations provide for the safe and efficient use of biosimilars in the clinical setting. The guidelines were built around findings from a survey issued by a task force within the NCCN’s Pharmacy Directors Forum, which began working on the project in 2019.
“There are significant challenges from an operational standpoint and unique risks that exist when using these products in the care of patients,” even though they have the potential to reduce the costs of health care, the NCCN said.
Risks of Using Biosimilars
Risks of using biosimilars include medication errors, financial toxicity for patients, and economic difficulties for health care institutions and providers. “Payer policies, such as single sourcing coverage of biosimilar medications, can be a root cause of some of these risks,” the group said.
The lower costs that biosimilars offer—approaching 50% in some cases—are enticing. “However, there are several important observations that must be considered which make biosimilar products less than the universal panacea they were hypothesized during their inception,” the NCCN guideline said.
Payers have a large role in the choice of biosimilars, and this was the top-rated concern among those who took the survey. Making the electronic health record work to identify coverage for biosimilars was the second highest-rated concern, followed by the challenges of obtaining payer authorization for biosimilars. Actual procurement of biosimilars and effective storage of multiple biologics also were high-ranking concerns.
“One strategy is to stock multiple biosimilar products…. However, this strategy may increase carrying costs and place a strain on storage requirements such as refrigerators,” the NCCN said. Dispensing errors may increase if staffers grab the wrong product off the shelves.
Payers may prefer one biosimilar over others potentially because they have a contract with the manufacturer rather than because it’s the best option for the health care institution or patient, the NCCN said.
“The payer will specify a particular biosimilar product as being the preferred product and that other biosimilars are therefore ‘not medically necessary’ or ‘do not meet medical necessity requirements’ unless or until the patient has received and is unable to tolerate the ‘preferred’ biosimilar product,” the NCCN said.
These payer preferences threaten the continued availability of biosimilars and add an element of complexity that can lead to costly errors for the practice that must follow different policies for multiple payers. “Such challenges are based solely on insurance company restrictions, which are generally not visible to the physician or pharmacist, and often are not known ahead of time by the finance department,” NCCN said.
The NCCN said such practices run counter to Congress’ intent when it established the biosimilars approval pathway to promote the development of lower-cost biologics. The NCCN recommended that health care providers “reach out to legislators, government agencies, employers and patients to help advocate against payer’s practices of allowing for reimbursement of only one biosimilar product.”
In the meantime, one coping strategy would be to carefully coordinate in-house activities at health care institutions, such as having pharmacy departments work closely with precertification departments and revenue cycle teams, the recommendations said.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
