- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
New Interactive Biosimilar Tool Can Help Educate Providers and Patients
Biosim.care is a new interactive education tool that can help patients, caregivers, and providers navigate decision-making and treatment conversations for multiple disease states.
PRIME Education has launched Biosim.care, a new education tool for providers, caregivers, and patients on all things biosimilars, including resources for different treatment spaces.
The free, interactive platform provides patients and providers with targeted, easy-to-navigate education materials covering a range of biosimilar topics, including basic information on biosimilars and their benefits, how to navigate insurance policies for biosimilars, and how providers and patients can talk to each other to foster better shared decision-making conversations.
Experts have long advocated for more patient- and provider-targeted biosimilar education efforts because they can play a major role in increasing adoption and acceptance of biosimilars, especially in the immunology space, where uptake for infliximab and adalimumab biosimilars have been lower than expected. For example, Cardinal Health’s 2023 report found that rheumatologists’ familiarity and confidence in biosimilars significantly increased between 2020 and 2022 in large part thanks to more effective provider-targeted education efforts.
Additionally, patient education efforts can help counter the nocebo effect, in which patients’ negative emotions about a particular product can lead to perceived loss of efficacy or increase in adverse events, often leading to treatment discontinuation. This can especially be an issue in the chronic disease space, where patients are wary of switching from a reference agent that they are comfortable with to a biosimilar version.
Prior to launch, the platform was reviewed by the Academy of Managed Care Pharmacy Foundation. The website is also supported by the Biosimilars Council, a division of the Association for Accessible Medicines. Although the platform is not sponsored by any particular pharmaceutical company, it was developed using educational grants from Coherus Biosciences, Fresenius Kabi, Biocon, Organon, and Pfizer.
The Biosim.care platform allows users to create their own CareGuide, in which modules can be saved for review. The platform is free to use and offers information for both patients/caregivers and providers.
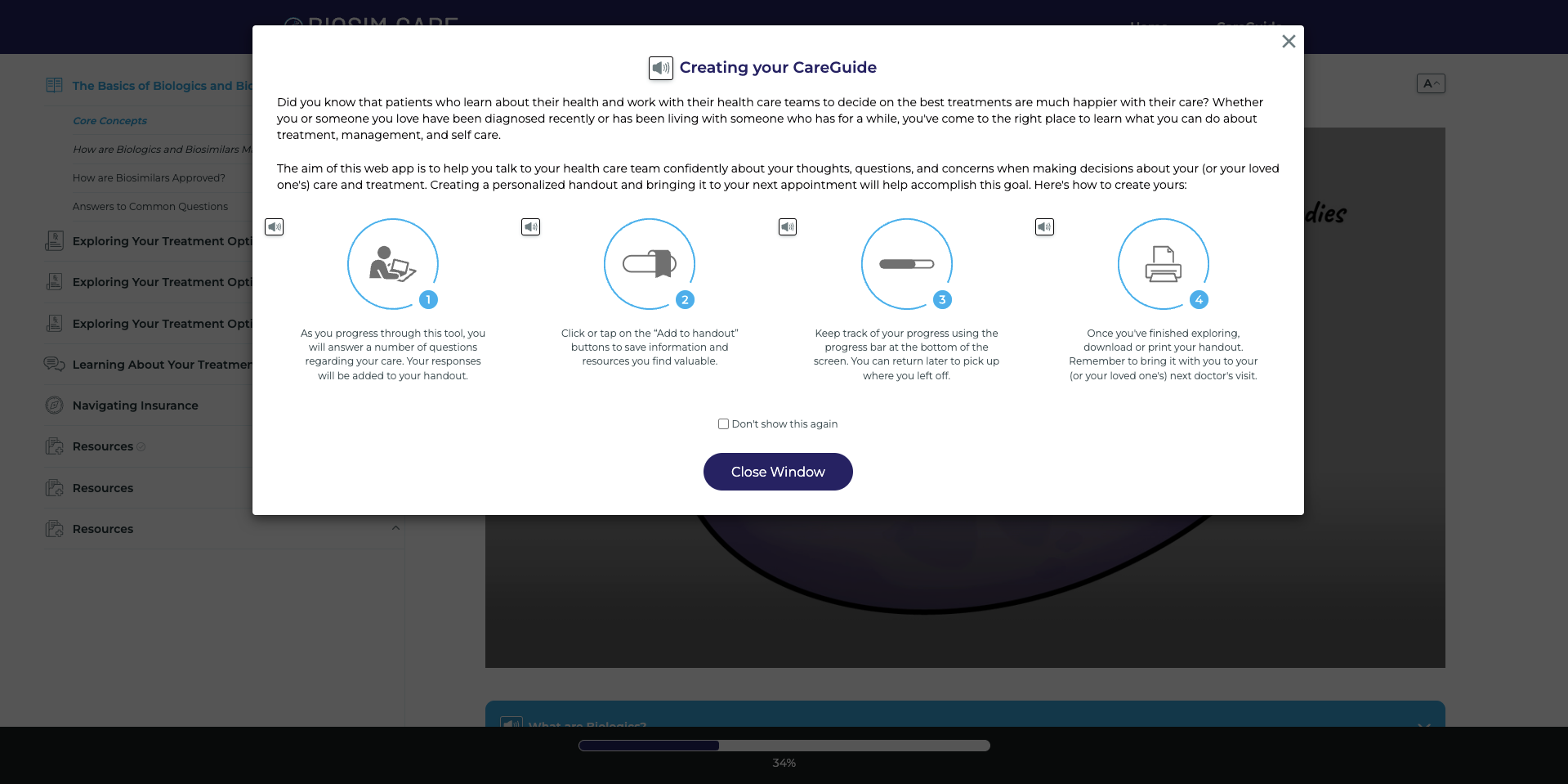
The homepage of the website allows for visitors to choose whether to view the patient/caregiver or provider portals. Visitors are then asked to indicate whether they want to learn more about rheumatology biosimilars, gastroenterology biosimilars, or oncology biosimilars. Depending on the disease space chosen, will determine which pages are available.
Visitors will be able to select different navigation paths, where they will be able to click through to learn more about a particular topic. The paths feature a sidebar so that visitors can switch between modules easily. The website also has accessibility features, including text to speech readers and font size adjustments. Patients can also monitor their progress at the bottom of the screen and save modules to their individual CareGuide to review at their convenience.
Visual elements such as tables and educational videos to help explain the basics of biologic drugs, biosimilars, and how these products are made and approved. Users can also learn about the specific of their biosimilar treatment options, including brand names, nonproprietary names, launch status, interchangeability labels, indications, storage requirements, and more.
For the “Navigating Insurance” path, users can gain insight into step therapy and prior authorization policies, the differences between Medicaid and commercial insurance, and advice on how to navigate the insurance process, including links to patient advocacy organizations and resources on patient assistant programs and co-pay cards.
The platform also offers additional educational resources, with links to the Biosimilars Patient Resource Center, how-to videos on self-injections, and government websites on understanding health insurance coverage. There are also recommendations for medication tracking apps for patients with chronic conditions.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
