- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Pfizer Scales Down Biosimilars Activity in China
Pfizer said it has made a business decision to cease production of biosimilars at a $350 million plant it built recently in Hangzhou, China.
Pfizer is offloading a biosimilars manufacturing plant in Hangzhou, China, to WuXi Biologics, backing out of a $350 million investment it began there in 2016 to produce biosimilars for the China market, Pfizer said in a statement.
“After a comprehensive review of the biosimilars market and the company’s global manufacturing network, Pfizer has made a difficult decision to halt its biosimilars programs in China and sell 100% of its equity interest in Pfizer Biologics (Hangzhou) Co. Ltd,” the company said.
The plant was envisioned as Pfizer’s first biotechnology center in Asia and incorporated what was described as advanced, flexible, single-use biomanufacturing technology that would allow rapid production and savings over existing processes of up to 50%. At the time of the announced construction, an opening was anticipated in 2018. Pfizer said at the time it was encouraged by a series of Chinese government reforms designed to stimulate the biologics industry. The factory was expressly intended for the production of biosimilars and other biologics and was expected to employ more than 150 workers.
China's Biologics Reform Engine
In recent years, China has made significant reforms designed to improve regulation of biosimilar and biologics development and also speed up review of product candidates to broaden access for the country’s population. The country has numerous biosimilars on the market, but none of them produced by foreign companies (Table).
Click to enlarge.
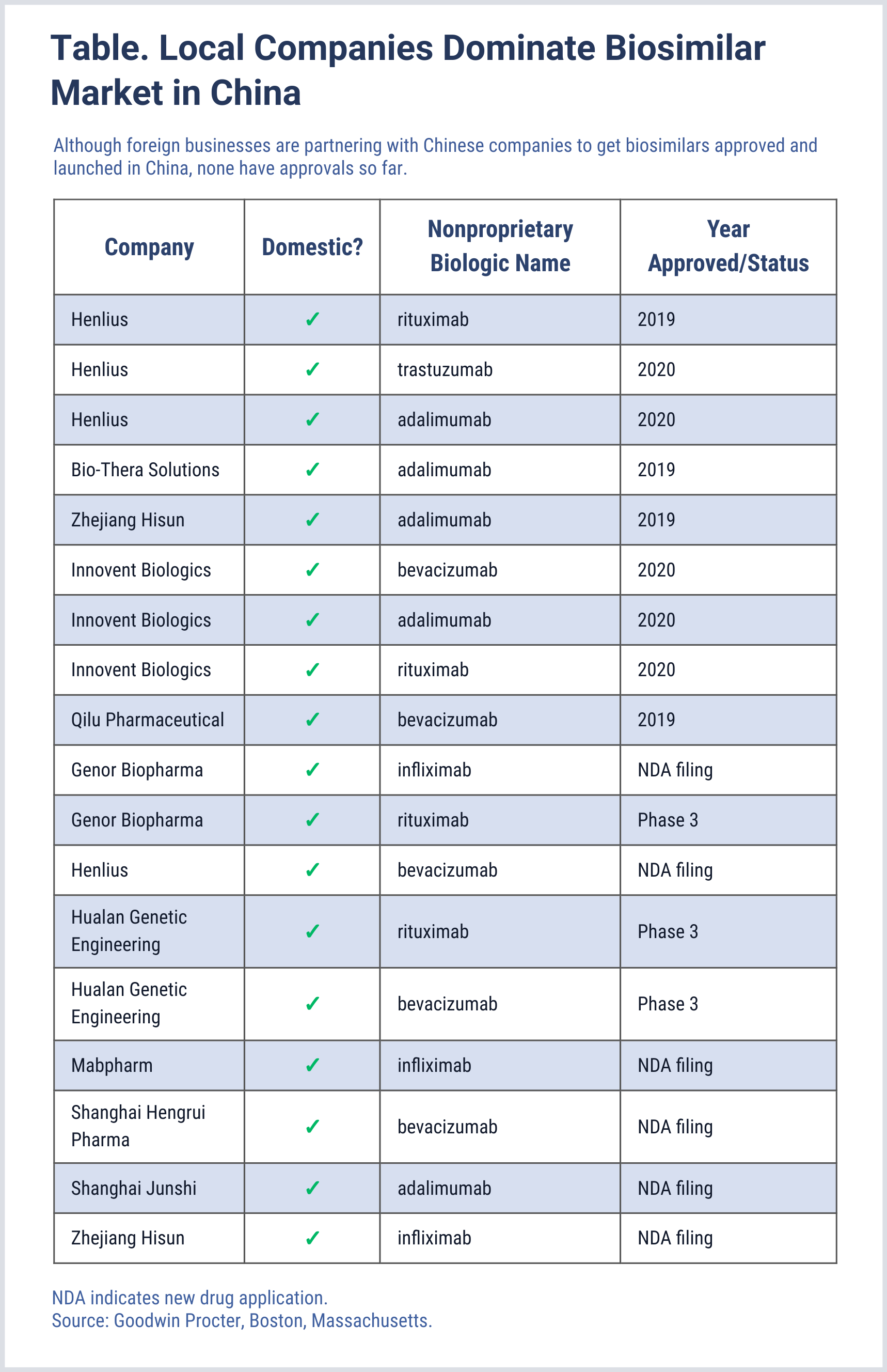
Pfizer’s operations at Hangzhou will terminate in the first half 2021, and Wuxi Biologics, which is based in Wu Xi City, China, will use the plant to manufacturer biologics for local and global markets. BioProcess said Pfizer will continue producing drugs for the China market.
In a statement, WuXi said it will acquire Pfizer’s labor force at the 50,000-square-meter Hangzhou plant, which contains a pair of 2000-liter single-use bioreactors and is equipped for filling vials and syringes for shipment in ready-to-use doses.
“We are pleased to work with Pfizer and add the new Hangzhou biologics manufacturing facilities to our global network to address the surging manufacturing demands for late-stage and commercial products,” said Chris chen, CEO of WuXi Biologics. “Globally, drug substance and drug product capacities are in urgent need now.”
Pfizer has enjoyed dramatic recent success with biosimilars, reaping $525 million in biosimilar income for the fourth quarter of 2020, up 86% from the final quarter of 2019. The company launched bevacizumab (Zirabev), trastuzumab (Trazimera), and rituximab (Ruxience) biosimilars in the United States in 2020.
The decision to spin off the Hangzhou plant contrasts with Pfizer’s 2020 decision to discharge its Upjohn division but retain its biosimilars unit.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
