- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Phase 3 Study Reports Similar Efficacy Between SB17, Stelara in Psoriasis
A phase 3, 28-week comparative clinical trial in patients with moderate to severe plaque psoriasis confirmed similarity of the proposed ustekinumab biosimilar SB17 (Samsung Bioepis) to the reference product (Stelara) in efficacy, safety, pharmacokinetics, and immunogenicity.
A phase 3, 28-week comparative clinical trial in patients with moderate to severe plaque psoriasis confirmed similarity of the proposed ustekinumab biosimilar SB17 (Samsung Bioepis) to the reference product (Stelara) in efficacy, safety, pharmacokinetics, and immunogenicity.
Image credit: SergeVo - stock.adobe.com
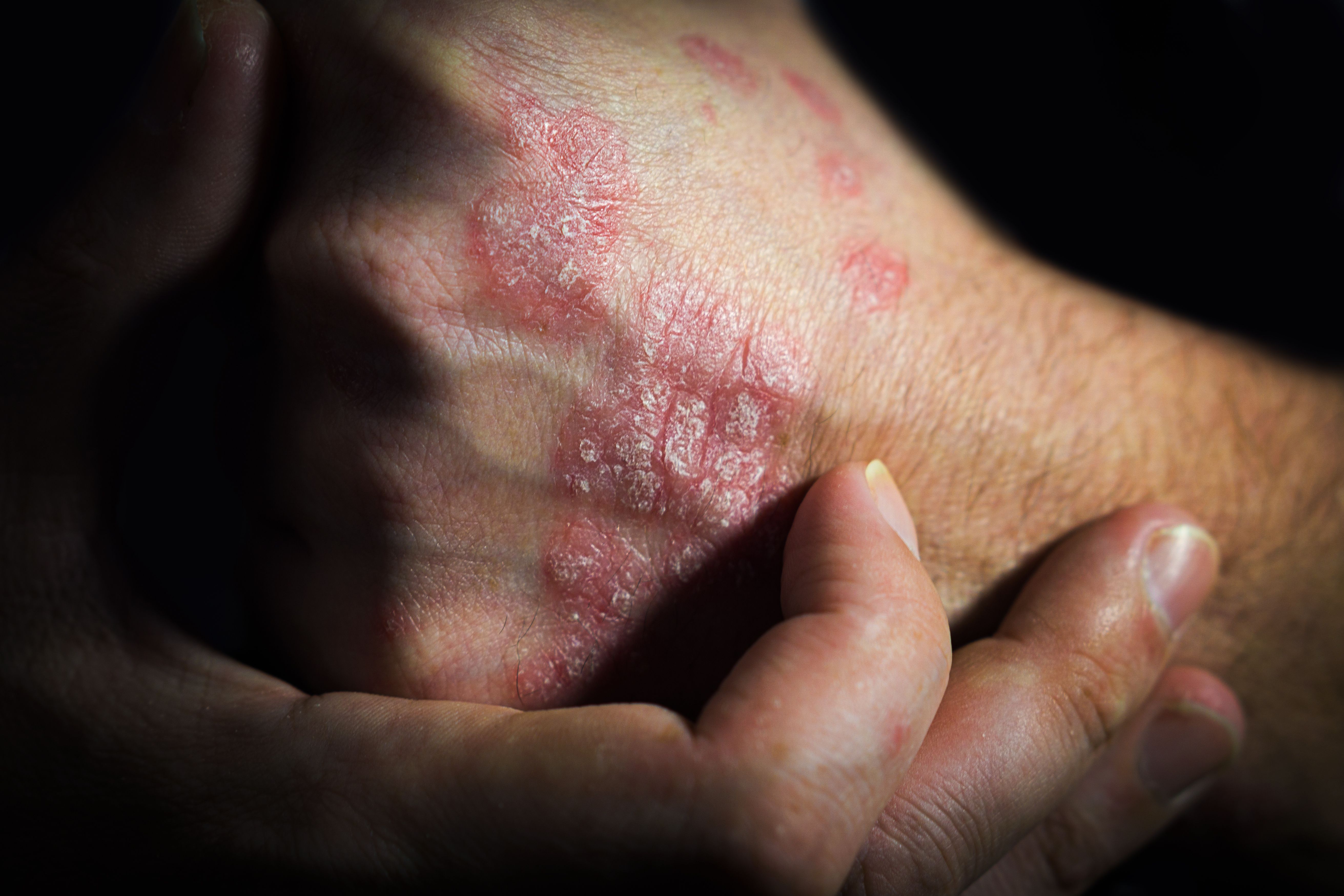
Most biologics for inflammatory diseases, including psoriasis, are inhibitors of tumor necrosis factor (TNF)-α, but ustekinumab is the first of newer biologics focused on other targets, the authors said. Ustekinumab, an inhibitor of interleukins 12 and 23, is used to treat psoriasis, psoriatic arthritis, and inflammatory bowel disease. The authors cited advantages of ustekinumab over anti-TNF biologics, such as less frequent dosing and “perhaps a better safety profile.”
The ustekinumab biosimilar candidate SB17 has previously been shown to be similar to the reference product in physicochemical characteristics and pharmacokinetics in healthy human subjects. The current study assessed clinical efficacy, safety, pharmacokinetics, and immunogenicity compared to reference ustekinumab for 28 weeks in moderate to severe plaque psoriasis.
The phase 3, double-blind, randomized controlled trial was conducted in 45 centers across 8 countries. Subjects (n = 249) were randomized to receive SB17 or the reference product at 0 weeks, 4 weeks, then every 12 weeks until week 40. The primary endpoint was percent change from baseline in Psoriasis Area and Severity Index (PASI) at week 12, and additional endpoints were measured through week 28.
The adjusted difference in PASI change from baseline at week 12 was –0.6% (95% CI, –3.780 to 2.579), within the predefined equivalence margin of –5% to 15%. The authors commented that the time-response curve of percent change of PASI from baseline was “nearly identical” between groups up to week 28. Secondary efficacy endpoints, including Physician's Global Assessment and Dermatology Life Quality Index were “also comparable” between the biosimilar and reference product groups.
Overall incidence of treatment-emergent adverse events (TEAEs) was 48% in the biosimilar group and 49% in the reference product group. According to the authors, most were mild to moderate and not considered related to the study drugs. The most frequently reported TEAEs were nasopharyngitis, COVID-19, and upper respiratory tract infections. Six participants (2.4%) in the biosimilar group and 3 (1.2%) in the reference product group experienced serious TEAEs, but none were considered related to the study drugs. There were no deaths, and 1 TEAE in the reference product group (hepatic steatosis) that led to discontinuation of the study treatment. TEAEs of special interest occurred in 28% of patients receiving the biosimilar and 30% of those receiving the reference product, and most were infections.
The pharmacokinetic profiles were “generally comparable” between groups, the authors said. Incidence of anti-drug antibodies was lower in the biosimilar group at 13%, compared to 39% in the reference product group, and incidence of neutralizing antibodies was 14% and 35%. However, the primary efficacy outcome was comparable between groups in ADA-positive and ADA-negative subgroups, the authors commented, adding that the difference in ADAs could be due to differences between the cell lines that produce the 2 monoclonal antibodies. They noted that similar differences in immunogenicity compared to the reference product have been cited for other ustekinumab biosimilars, and “lower immunogenicity does not preclude biosimilarity if the ADA-negative subgroups of the biosimilar and the originator have similar efficacy.”
The author concluded that SB17 was clinically similar to the reference ustekinumab up to week 28, with comparable safety and pharmacokinetic profiles.
Reference
Feldman SR, Narbutt J, Girolomoni G, et al. A randomized, double-blind, phase III study assessing clinical similarity of SB17 (proposed ustekinumab biosimilar) to reference ustekinumab in subjects with moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2024;91(3):440-447. doi:10.1016/j.jaad.2024.04.045
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
