- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Physicians Urge FDA to Waive Interchangeability Standard for Insulin Biosimilars
Two doctors call for interchangeability requirements for biologics to be waived for insulin products under the new regulatory pathway under the Biologics Price Competition and Innovation Act (BPCIA) of 2009.
The new biologics pathway for insulins could complicate the process of getting a greater supply to patients in need, according to a position paper in the New England Journal of Medicine. Two doctors called for biosimilar insulins that meet the basic tests for approval to automatically be granted interchangeability without the need for immunogenicity testing.
The authors noted that “the FDA guidance is a reminder that the rules of the [Biologics Price Competition and Innovation Act (BPCIA) of 2009] should be carefully reinterpreted in order to maximize benefit, affordability, and access to insulin for all Americans with diabetes.” They note that the FDA has indicated an interest in streamlining the approval process for biosimilar insulins by removing the interchangeability requirement.
For a patient with type 1 diabetes, the cost of insulin in the US market nearly doubled from 2012 to 2016, from $2864 to $5705, while the overall cost, including ancillary care, rose from $12,467 to $18,494 during that time, according to a study by the nonprofit Health Care Cost Institute published in early 2019 (Figure).
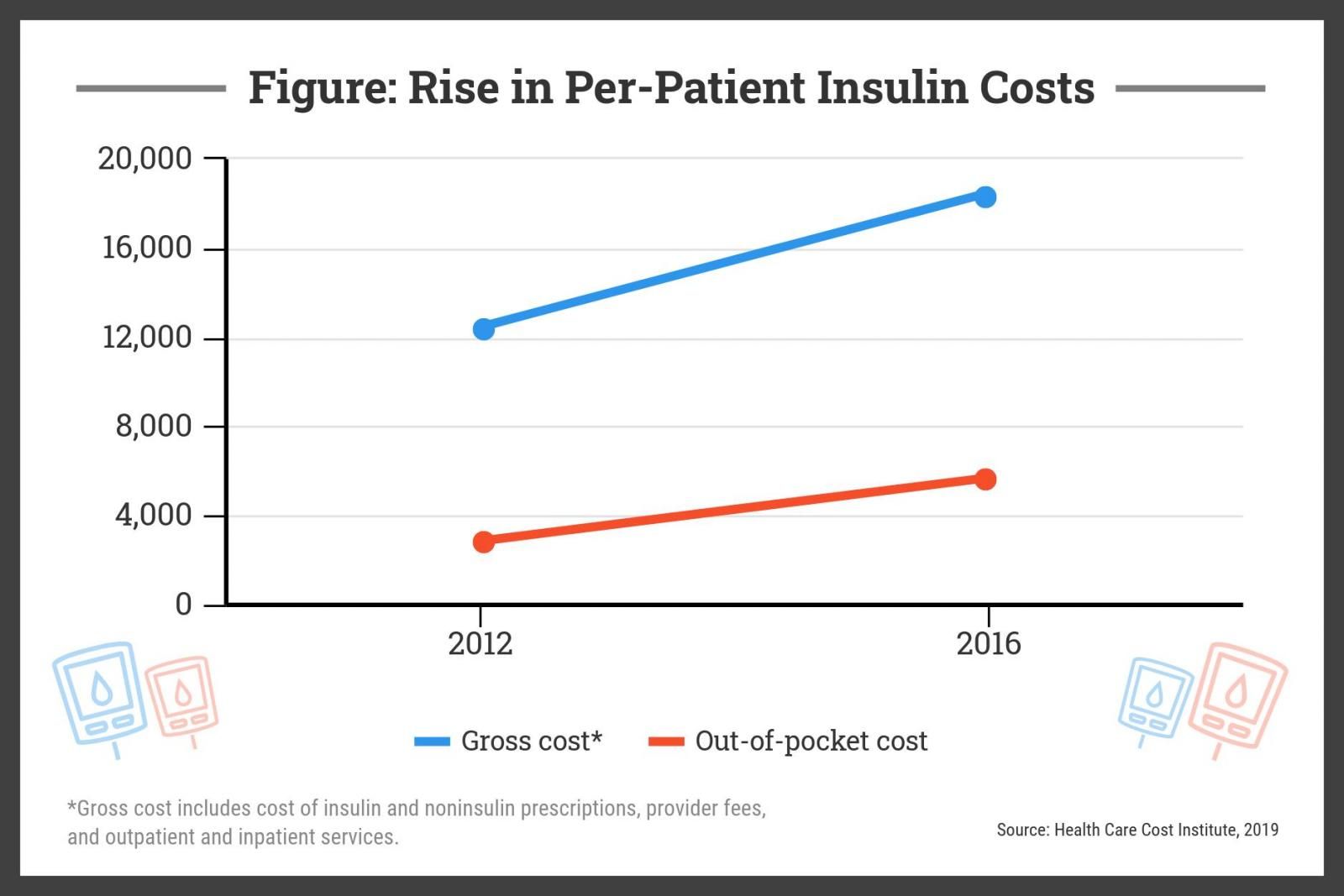
“This policy could play a major role in enabling robust generic competition and ensuring access to high quality, affordable insulin,” Mariana P. Socal, MD, PhD, and Jeremy A. Greene, MD, PhD, wrote.
Under the current provision in the BPCIA, certain “small molecule” drugs previously approved as generics, including insulins, may be approved under the biologic regulatory pathway, but insulin biosimilars would be subject to the same rules for demonstrating interchangeability with reference medicines as other types of biosimilars.
“Regulation of insulin products has become more complicated” amid the growth of the biotech industry and the establishment of new biologic approval pathways, the authors said.
Under the small molecule pathway, proof of similarity was considered proof of interchangeability, which means pharmacists could use non-reference products without consulting physician prescribers. However, switching insulins to the biologic pathway means that biosimilar insulin manufacturers will have to conduct extra clinical trials to prove interchangeability with existing insulin products.
Years of Evidence Justify a Smoother Pathway
The authors argue that years of experience with insulins approved as generics indicate they can be safely switched without these immunogenicity tests, and further, they contend, “the complications and death caused by lack of affordable insulin are a more material threat to public health and welfare than potential differences in immunogenicity profiles” making the exception all the more critical.
Although insulin products consist of just 50 amino acids (compared with large molecule agents, which can consist of about 1300), insulin products are able to be called biologics because they require a genetically modified living organism, usually yeast or bacteria, for production.
The policy of granting interchangeability upon proof of bioequivalence has been shown to yield average price reductions of 80% within 5 years after introducing generic alternatives to the market. Insulin products currently have this designation and may lose it with the transition to the biologics approval pathway, which will make switching products to save money much more difficult for patients, the authors write.
“We believe it is crucial for an exception to be made for insulin products such that proof of similarity will be considered proof of interchangeability [under the biologics pathway],” the authors wrote.
Lowering barriers for market entry and reducing drug-development costs by forgoing extra clinical trials would promote competition among insulins and lower drug prices would help make the US insulin supply more secure, the authors said. The rise in insuling costs has been attributed to higher insulin prices in general and a shift toward more expensive insulin products.
“These benefits would accrue even if the [BPCIA pathway revision] is met with opposition from reference-product manufacturers, including those that don’t manufacture insulin but may be concerned about the precedent-setting nature of this exemption,” the authors said.
Fewer Problems With Insulin Products
One of the reasons the authors feel that interchangeability requirements should be waived is that the interchangeability difficulties for large molecule drugs, such as the potential for immunogenicity and the loss of efficacy, have not been observed with insulin products.
Additionally, antibodies that can develop for insulin users have not been observed to be associated with worrisome clinical outcomes such as changes in glucose control or dose requirements, hypoglycemia, or long-term diabetic complications, they said.
“Any harms that might stem from considering biosimilar insulins to be interchangeable with reference insulin products remain entirely theoretical,” the authors noted.
In fact, the American Diabetes Association guidelines “already acknowledge that insulin products within the same range of therapeutic onset and duration of effect…are similarly effective and can be selected at the physician’s discretion.”
Although, patients with diabetes may prefer certain products or brands, clinical research supports equivalence among treatments. Changes in insurance policies have also led to large-scale switching between insulin products, the authors note.
Another reason for an interchangeability exception for insulin is that if problems do occur, patients with diabetes will easily and quickly be able to tell. “If a biosimilar insulin product didn’t provide an adequate clinical effect, patients could identify this failure within an hour and correct it,” the authors said.
The nature of diabetes with the constant management of blood glucose levels, “mitigates any possibility of clinical failure going unnoticed.” Issues with other biologics could take months or years to notice and even when they are found, significant damage may already have been done, they said.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
