- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Prince Edward Island Announces New Biosimilar Switching Policy
Prince Edward Island became the eleventh jurisdiction in Canada to implement a biosimilar transition policy, in which patients who are currently taking select reference agents will transition to biosimilar versions.
The Government of Prince Edward Island (PEI) became the eleventh Canadian jurisdiction to implement a biosimilar “switching” or transition policy, according to a statement from Biosimilars Canada.
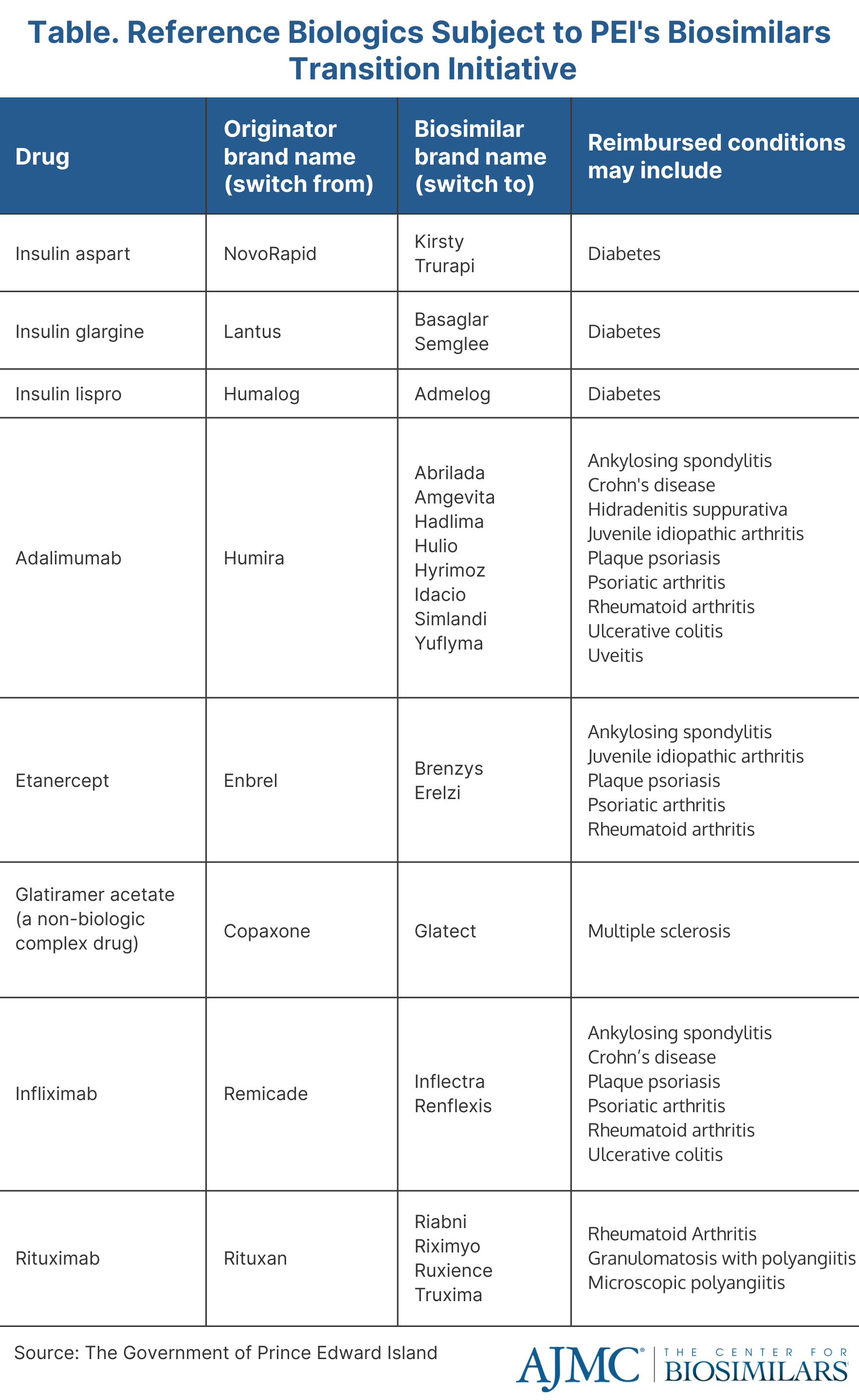
The policy will require patients enrolled in the PEI Pharmacare program who are taking certain reference biologics to transition to a biosimilar version (Table). Additionally, all new patients in need of therapy using those agents will be required to start on a biosimilar. The new policy will allow PEI to fund more new drug therapies, bring innovation to the province’s health care system, and improve patient care.
If a patients is unable to get an appointment with their prescriber before the deadline, PEI's government website offers an exception form for providers to fill out. Once approved, the special authorization for the reference agent will be extended for 1 month following the patient's upcoming appointment. The appointment will need to be scheduled before September 30, 2024.
“Biosimilars Canada and its member companies congratulate Health and Wellness Minister Mark McLane and the Government of Prince Edward Island for implementing a biosimilars switching policy to help support the long-term sustainability of the province’s public drug program and health care system,” said Jim Keon, president of Biosimilars Canada.
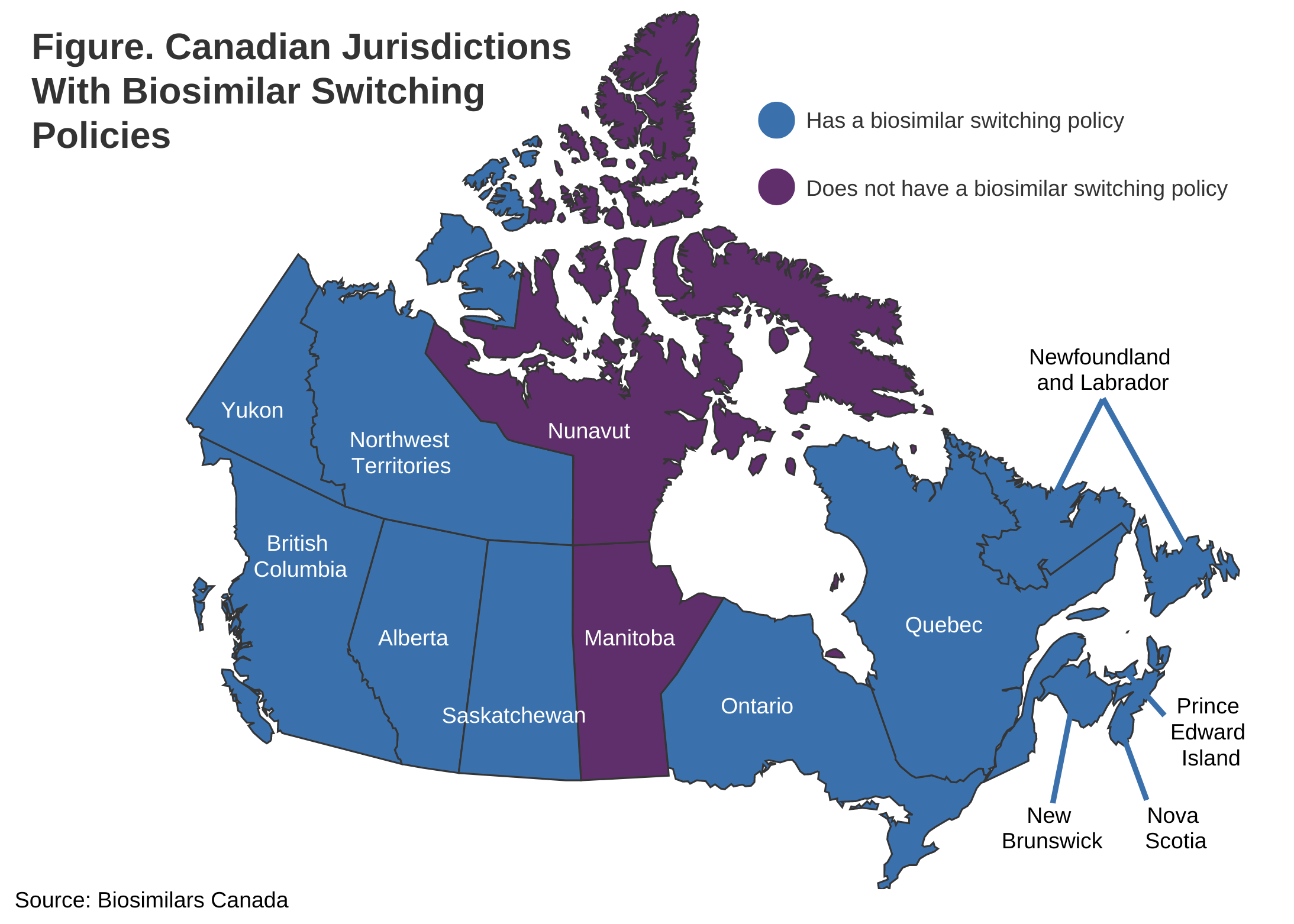
PEI’s policy follows in the footsteps of British Columbia (May 2019), Alberta (December 2019), New Brunswick (April 2021), Quebec (July 2021), the Northwest Territories (December 2021), Nova Scotia (February 2022), Saskatchewan (October 2022), Ontario (December 2022), Newfoundland and Labrador (March 2023), and the Yukon (March 2023) (Figure).
Providers have until June 30, 2024, to tell their patients on the selected reference agents that their product will change and educate them on what a biosimilar is and the safety of transitioning. After the deadline, the reference medicines will no longer be covered under the public health plan. As new biosimilars become available, the initiative will apply to other originator biologics listed on the PEI Pharmacare formulary.
Manitoba and Nunavut are the only juridictions that do not have biosimilar transition policies.
The topic of switching or transitioning patients from a reference product to a biosimilar has raised concerns about whether product transitions will alter safety or efficacy outcomes. However, no province with a switching policy has experienced impacts on clinical outcomes and both the European Union and United Kingdom have declared all biosimilars as interchangeable with their reference products.
In Health Canada’s biosimilar fact sheet, the agency emphasizes that “patients and health care providers can have confidence that biosimilars are effective and safe for each of their authorized indications, and that no differences are expected in efficacy and safety following a change in routine use between a biosimilar and its reference biologic drug in an authorized indication.”
Although biosimilars have helped to revolutionize the treatment of several chronic and oncologic conditions, they can cost $10,000 to $25,000—in some cases, even more—to treat a patient for a year, which can place an enormous financial burden on health systems and strain on drug budgets.
Manitoba and Nunavut are the remaining jurisdictions without a biosimilar transition policy. However, Manitoba is the only jurisdiction left that has a public health plan.
Nunavut is the smallest of the territories—it has a population of about 38,000 residents—and a large portion of residents are covered by the federal drug programs. Although it does not have the resources to maintain its own formulary and does not provide prescription drug coverage for uninsured residents, Nunavut provides reimbursement for the products that are covered by the federal Non-Insured Health Benefits program formulary. Due to the jurisdiction’s unique circumstances, Nunavut will likely never need a biosimilar transition policy.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
