- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Psoriasis Biosimilars Saved VA $67 Million in 2023
The Department of Veterans Affairs (VA) saved $67 million in 2023 through its strong adoption of biosimilars to curb the high costs of biologic therapies to treat chronic disease.
By embracing psoriasis biosimilars, the Department of Veterans Affairs (VA) successfully slashed over $67 million in costs in 2023, proving that these lower-cost biologics can deliver on promised cost savings; however, greater education is needed to unlock their full potential, according to an analysis.
The review, published in the Journal of Dermatologic Treatment, sought to clarify the clinical outcomes and cost shifts associated with patients either beginning treatment on or switching treatment to a biosimilar. Additionally, most indications for a biosimilar are extrapolated upon regulatory approval, causing concerns about whether the product performs as well in indications the biosimilar was not tested in. In the case of biosimilars for psoriasis, most of the approvals have been based on data in patients with rheumatoid arthritis.
Researchers chose to study biosimilar use within the VA health care system because it has increasingly adopted biosimilars since 2015 to curb the high costs of biologic therapies for chronic conditions like psoriasis. The researchers aimed to evaluate the real-world effectiveness of biosimilars in improving access to care while maintaining efficacy and safety. Additionally, the VA’s large and diverse patient population provided the authors with valuable insight into how biosimilars influence treatment outcomes and resource allocation within an integrated health care system.
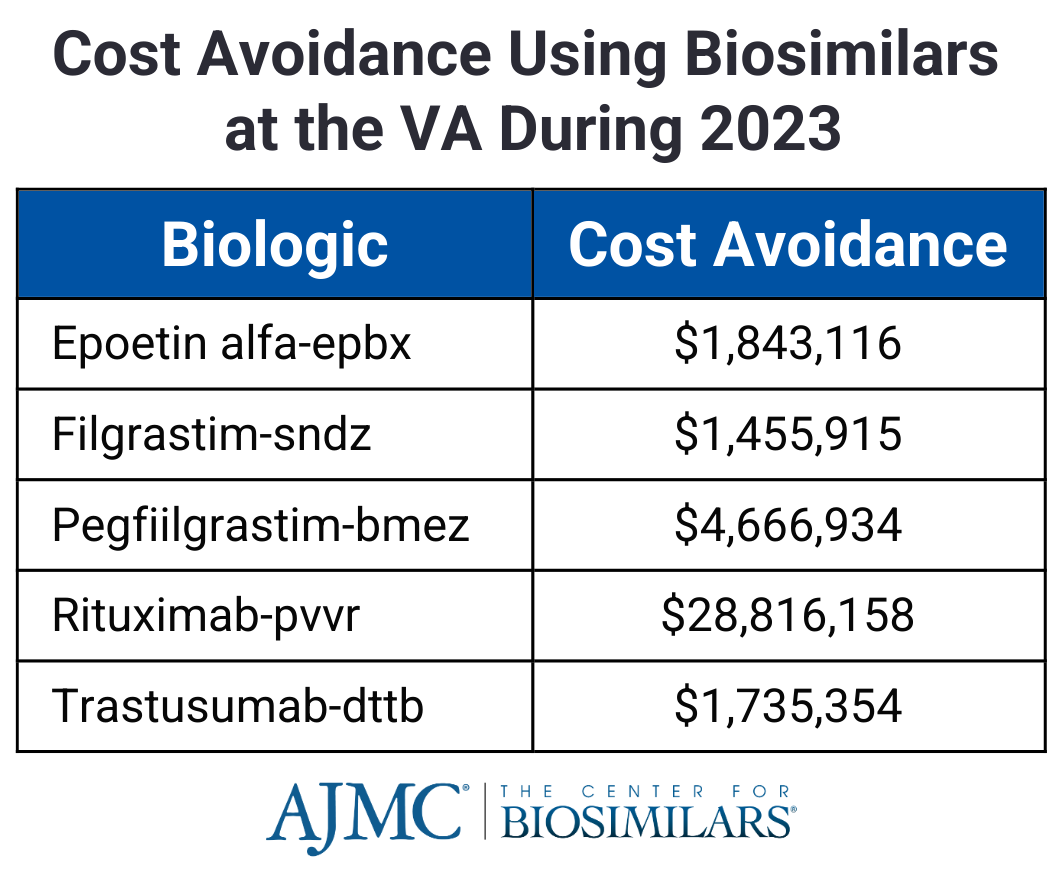
The review also explored the regulatory challenges, approval processes, and clinical considerations, such as interchangeability, switching, and extrapolation of indications, to highlight how biosimilars can enhance patient access to biologic treatments without compromising therapeutic goals.
Researchers conducted a systematic literature search using PubMed, Wright State University Libraries, and Google Scholar. The initial search concluded that tumor necrosis factor-α inhibitor biosimilars—such as those for Humira (adalimumab), Enbrel (etanercept), and Remicade (infliximab)—generally have similar effectiveness and safety compared with their originators in patients with psoriasis. However, treatment response can vary based on psoriasis type, with some forms responding better than others. For instance, an infliximab biosimilar showed varying response rates between plaque and pustular psoriasis.
Additionally, patient characteristics, such as gender and comorbidities, influenced switching from originators to biosimilars. Non-medical switching (economic or treatment availability reasons) was also discussed, with surveys revealing concerns among physicians, especially when patients were stable on originators.
Biosimilars Savings Are Contingent on Pricing Strategies
Researchers found data showing that adalimumab biosimilars can provide significant savings, but these are contingent on pricing strategies. In Denmark, a mandatory switch to adalimumab biosimilars led to substantial cost reductions. However, biosimilar adoption should not compromise treatment goals, and implementation costs (eg, education programs) must be considered.
Additionally, a study in British Columbia, a Canadian province with a policy that required all patients on certain biologics in 2019 to switch to biosimilar versions, showed no negative health impacts from nonmedical switching to psoriasis biosimilar.2 The program also generated $732 million in savings over 5 years for the province’s public health plan.
In the US, the VA has seen major cost savings from biosimilars, particularly with the infliximab biosimilar Renflexis, which saved over $28 million in 2023.1 However, the VA sets high discount thresholds for biosimilar adoption, and etanercept and adalimumab biosimilars are not yet in use. Expanding biosimilar utilization could further optimize the VA’s drug budget and improve access to treatments.
Studies on drug survival and biosimilar safety found that most patients on an adalimumab biosimilar remained on treatment after a year, with sustained skin improvements for up to 48 months. Additionally, patients who started treatment on a biosimilar have better clinical outcomes compared with those who switched from an originator.
Switching for nonmedical reasons led to higher drug survival (74%) than switching for medical reasons (24%). A study in Denmark found no major differences in treatment continuation after a required switch to biosimilars. Multiple treatment switches did not impact effectiveness or immune response.
Regarding safety, most side effects were mild, such as infections and injection reactions. No severe issues like cancer or death were reported. Infusion reactions were more common in patients with severe disease or allergies but could be managed. There were no cases of tuberculosis reactivation. The review authors noted organizations like the Biologics and Biosimilars Collective Intelligence Consortium that conduct continuous safety monitoring for biosimilars, helping to ensure pharmacovigilance over time.
Despite growing evidence supporting biosimilars, many providers remain hesitant due to concerns about education, prescribing autonomy, and patient trust. Surveys showed that 63% of US physicians feel uninformed about biosimilars, and 75% worry that forced switching could harm the provider-patient relationship. Patients also expressed concerns about safety, efficacy, and side effects, though many are open to biosimilars if properly informed. To increase adoption, the authors emphasized that better education on biosimilars, clear communication about their use, and ongoing pharmacovigilance will be essential.
Higher discontinuation rates were observed in patients who switched from originator biologics to biosimilars, with the nocebo effect playing a key role. Differences in injection devices and excipients can also affect tolerability. Effective patient education, clear communication, and provider support are crucial to improving biosimilar acceptance and adherence.
The authors concluded, “It is necessary we continue to monitor biosimilars for efficacy, drug survival, and safety, as well as continue to monitor and address sources of concern to promote compliance with biosimilars. Additional education of providers about the approval and use of biosimilars in psoriasis can continue to aid with making informed treatment decisions.”
References
- Reese R, Nanavath SR, Martin J, Travers JB, Rohan CA. A review of biosimilars in psoriasis: impacts on efficacy, safety, access, and a first-hand look at biosimilar cost savings within the Department of Veterans Affairs. J Dermatolog Treat. 2024;35(1):2402912. doi:10.1080/09546634.2024.2402912
- Jeremias S. British Columbia’s biosimilar switching program saves $732 million in 5 years. The Center for Biosimilars®. June 18, 2024. Accessed February 11, 2025. https://www.centerforbiosimilars.com/view/british-columbia-s-biosimilar-switching-program-saves-732-million-in-5-years
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
