- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Study: Biosimilar Use Varies by Type of Institution and Physician Characteristics
Physician specialty and practice setting each play a significant role in whether biosimilars are administered vs originators, according to a large, cross-sectional study.
A patient in a hospital outpatient setting was 42% less likely to receive a filgrastim biosimilar than a patient in an office setting, but 73% more likely to receive an infliximab biosimilar, according to a new study examining the relationship between biosimilar use and practice setting and patient/physician characteristics.
Clear differences in filgrastim biosimilar administration according to practice setting were noted in the study of Medicare fee-for-service beneficiaries who received either filgrastim (n = 25,870) or infliximab (n = 14,786) products from the launch of these biosimilars through December 2018.
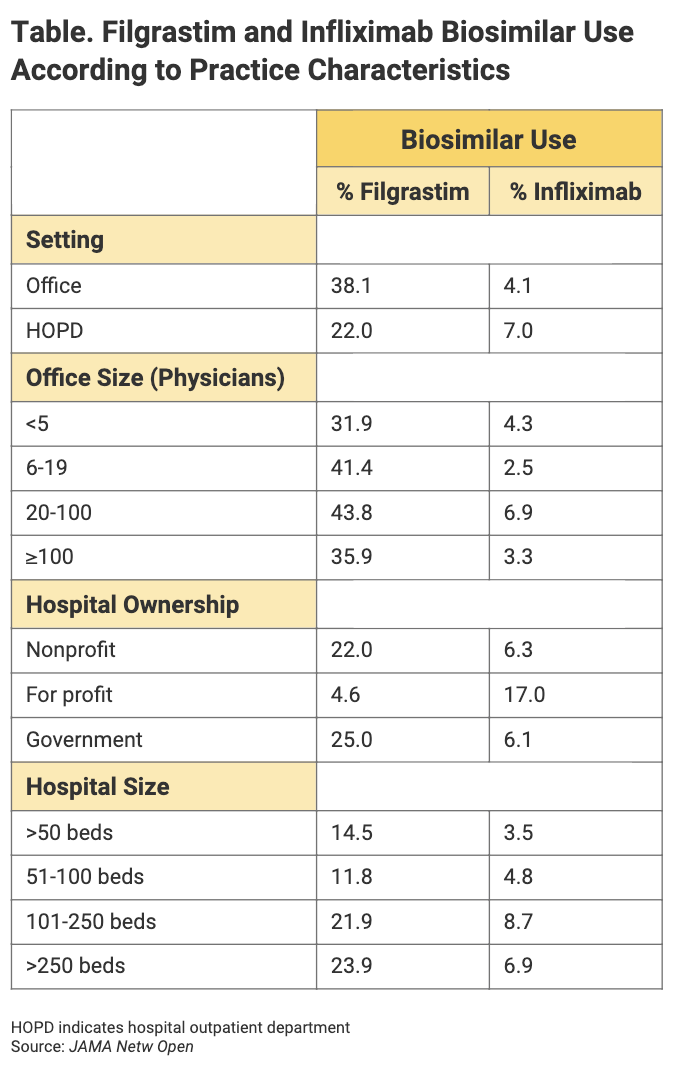
High prescribing volume by individual physicians was a factor associated with frequency of biosimilar administration, as was specialty. Practice characteristics that were associated with biosimilar use included setting and hospital ownership status (Table). Patient characteristics were only “weakly associated” with biosimilar use, the authors said.
Click to enlarge.
“Importantly, this study demonstrates that factors associated with biosimilar use may differ across drug classes, and therefore, each drug class may require different interventions to promote use,” the authors concluded. The findings also suggest that practice types play a role in steering physicians toward biosimilars.
Filgrastim Use
Separate figures were provided for filgrastim vs infliximab biosimilar use.
For filgrastim, the investigators examined physician time in practice but found no major differences in terms of biosimilar prescription. The same applied for provider gender. Using primary care specialists as the reference, investigators found that hematologist/oncologist practitioners were 3 percentage points less likely to prescribe filgrastim biosimilars, and oncologists were 3.4 percentage points less likely to do so.
Using physicians in independent practices as the reference, investigators found that physicians practicing in hospital-owned practices were 3.1 percentage points less likely than their counterparts to prescribe filgrastim biosimilars.
But when it came to practice characteristics, using office-based practices as the base, investigators found that hospital outpatient departments were 16.1 percentage points less likely to prescribe filgrastim biosimilars.
Office size was also a factor. Using those with less than 5 physicians as the reference, investigators found that practices with 6 to 19 physicians were 9.4 percentage points more likely to prescribe filgrastim biosimilars; and practices with 20 to 100 physicians, 11.9 percentage points more likely.
Among hospitals, using nonprofit institutions as the reference, for-profit hospitals were 17.4 percentage points less likely to prescribe filgrastim biosimilars, and government institutions were 3 percentage points more likely to prescribe them.
Hospitals with greater than 250 beds were 9.4 percentage points more likely to prescribe filgrastim biosimilars than those with less than 50 beds. Institutions with 101 to 250 beds were 7.4 percentage points more likely to prescribe filgrastim biosimilars.
Infliximab Use
According to the study, no significant differences in infliximab biosimilar use were noted between various types of practitioner, such as dermatologists, gastroenterologists, and rheumatologists. Similarly, no large differences were seen in infliximab use between independent and hospital-owned practices. HOPDs were 3 percentage points more likely to prescribe infliximab biosimilars than office-based practices. And for-profit hospitals were 10.8 percentage points more likely to prescribe infliximab than nonprofits.
“As this work demonstrates, adoption of biosimilars in Medicare has been uneven across different products. The uptake of filgrastim has risen consistently over time, reaching 52% of the market by December 2018. However, the uptake of infliximab biosimilars has been significantly slower, reaching 10% of the market by December 2018,” the authors wrote.
The authors speculated that the reasons for slower uptake of infliximab biosimilars could be an unwillingness among physicians to switch patients to biosimilars who are doing well on the originator brand. They said that financial incentives for infliximab and its biosimilars may be different than for the originator brand. “The unique contracting mechanisms used by the manufacturer of originator infliximab (eg, Johnson & Johnson) have included exclusionary contracts and purchasing bundles with health care professionals, health care systems, and insurers to allegedly block biosimilar competition,” they wrote.
A shortcoming of the study was that it examined only Medicare patients and left out those who have commercial coverage, “for whom uptake patterns could be different,” the authors said. The study also was not designed to establish causal links between the various characteristics of institutions, patients, and physicians that might affect biosimilar use.
“Surprisingly, the direction of the associations between practice setting and uptake was opposite for the 2 drug classes,” the authors concluded. They recommended more investigation to understand the differences in use patterns more clearly.
Reference
Dean EB, Johnson P, Bond AM. Physician, practice, and patient characteristics associated with biosimilar use in Medicare recipients. JAMA Netw Open. Published online January 27, 2021. doi:10.1001/jamanetworkopen.2020.34776
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
