- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Study: Nocebo Is Likely Cause for Reverse Switching in IBD
Three-fourths of patients who reverse-switched to originator infliximab from CT-P13 benefited, although it wasn't clear why the biosimilar treatment "failed."
Among patients who switched from originator to biosimilar infliximab (CT-P13) and then back to the originator, 73.3% benefited from the reverse switch, although investigators postulated that the nocebo effect probably played a large role in the reasons for switching and outcomes.
Their study enrolled 758 patients with Crohn disease or ulcerative colitis, and reverse switching occurred in 75 patients (9.9% over 52 weeks). The investigators said this occurred mostly among women patients (70.7%) and female sex was a predictor for infliximab drug discontinuation, which they said was corroborated by findings from other studies.
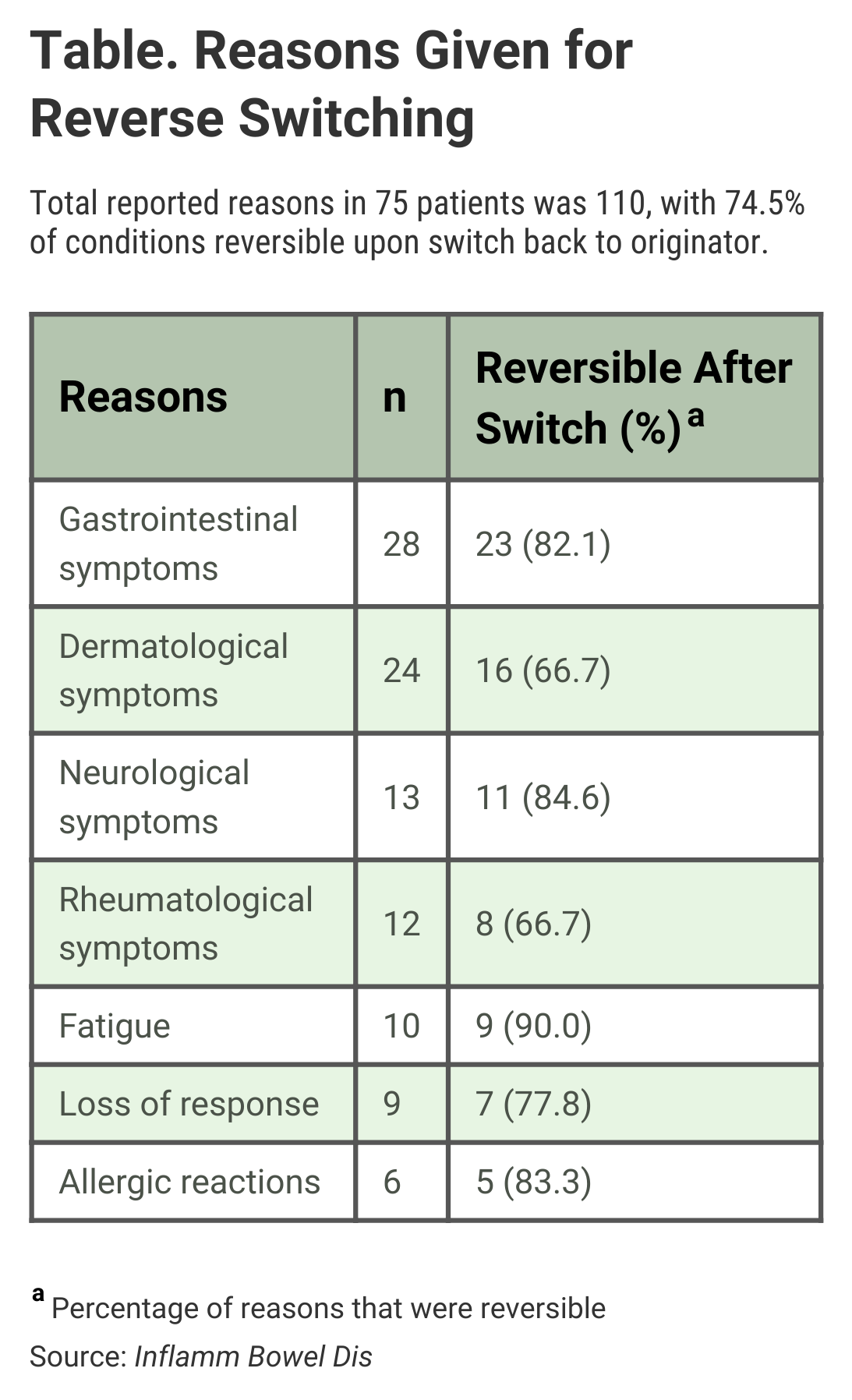
The authors wrote that gastrointestinal symptoms (25.5%) and dermatological adverse events (21.8%) were the most common reasons for reverse switching, but said “no clinically relevant differences were seen between the pharmacokinetics of CT-P13 and originator infliximab.”
For 9 of the 75 patients (12%), loss of response to the biosimilar was given as the reason for reverse switching, and 7 (77.8%) of those patients regained response once they resumed treatment with the originator drug (Table). “Infliximab persistence was equal between patients who were reverse-switched and those who were maintained on CT-P13,” the investigators said.
Click to enlarge
The 9.9% rate of reverse switching in the study, which was retrospective and involved data collected from multiple institutions (May 2015-December 2017), was slightly lower than for other studies of the same phenomenon (12.9% and 16.5%), although in all of the studies, the “clinical motivations for reverse switching were predominantly a perceived loss of response or adverse effects,” the authors said.
Besides the nocebo effect, which involves patients whose preconceptions about the effectiveness of a drug contribute to an apparent lack of efficacy, symptoms may have been falsely attributed to the biosimilar by patients and providers, the authors theorized. The nocebo effect is well documented, and “the majority of IBD [inflammatory bowel disease] patients have doubts and concerns about the safety and effectiveness” of drugs for IBD, the authors wrote.
The rate of reverse switching may have been partly attributable to the lack of clear policies in the treatment centers on how to manage patients with negative outcomes following the initial switch from originator to biosimilar, they said. “Thus, it is likely that a physician’s willingness to reverse-switch also contributed to this variation.”
One observation that mystified investigators was the occurrence of new or worsening skin reactions among patients who were reverse-switched. The blockade of tumor necrosis factor “is the mechanism considered to induce remission and is also thought to be the main immunological pathway involved in the pathogenesis of infliximab-related skin reactions,” so how skin reactions could occur in patients who did not benefit from the infliximab biosimilar is unclear, they said.
The authors wrote that patient expectations can be tempered by communication between a patient and a provider, or the provision of “tailored information.” Prior conditioning, psychological characteristics, and “situational influences” also contribute to the nocebo effect, they wrote. They concluded that nocebo notwithstanding, switching back to the originator product “seems effective” in addressing worsening gastrointestinal symptoms or loss of response.
Switching from the originator to infliximab biosimilar is commonplace in Europe, and it has been reported that 52 weeks after switching, the rate of patients discontinuing treatment because of loss of response, new adverse events, or a reverse-switch to the originator is from 7.5% to 29%, the authors said.
CT-P13 is distributed as Inflectra in the United States and Remsima in the European Union. The originator brand is Remicade.
Reference
Mahmmod S, Schultheiss JPD, van Bodegraven AA, et al. Outcome of reverse switching from CT-P13 to originator infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. Published online February 4, 2021. doi:10.1093/ibd/izaa364
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
