- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Study: Rapid Adoption Noted for Kanjinti During First Year of Availability
Investigators observed early adoption of the trastuzumab biosimilar Kanjinti in patients with early breast cancer and metastatic breast cancer.
Early adoption of the trastuzumab biosimilar Kanjinti was high among patients with breast cancer whether or not they had prior use of the originator (Herceptin), investigators said in a poster presentation at the National Comprehensive Cancer Network (NCCN) Virtual Annual Conference 2021.
Investigators set out to measure how quickly patients with a breast cancer diagnosis were started on the biosimilar during the agent’s first year of availability. Kanjinti, an Amgen product, was launched in July 2019. They used Flatiron Health retrospective data to develop 2 patient cohorts: those with early breast cancer (eBC, n = 2149) and those with metastatic breast cancer (mBC, n = 799). Those cohorts were further subdivided into patients with prior reference product (RP) exposure and no RP exposure.
Investigators said that among trastuzumab-naïve patients, the median time from diagnosis of eBC to initiation of biosimilar trastuzumab was 34 days; the respective time for those with a diagnosis of mBC was 30 days.
Among patients with prior RP exposure, 81% of patients with eBC transitioned and 75% with mBC transitioned to the biosimilar within 28 days of the final administration of RP. “Use of [Kanjinti] was observed in both RP-naïve and prior RP-treated patients, with evidence showing comparable patient characteristics between the 2 groups,” investigators wrote (Table).
Click to enlarge.
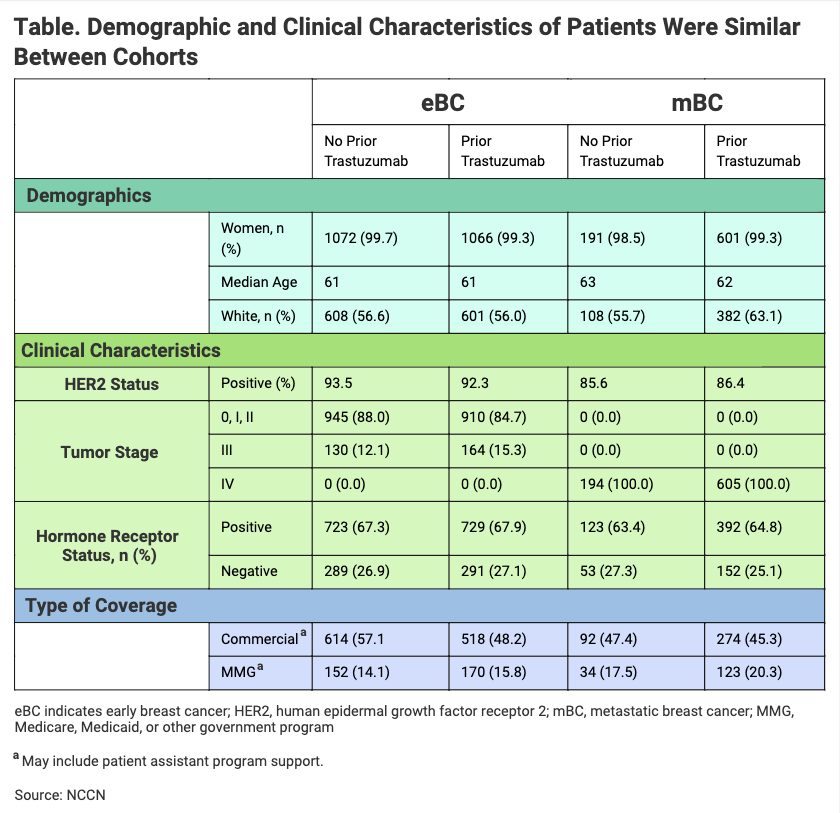
The investigators found that the first providers began to employ the trastuzumab biosimilar soon after it was launched—within 4 and 12 days of market entry. In the United States, Kanjinti was the first trastuzumab biosimilar to be launched, although it was not the first trastuzumab biosimilar to be approved by the FDA.
The study did not evaluate for reasons why patients previously treated with RP were transitioned to the biosimilar, owing to a lack of appropriate data, investigators said.
Reference
Jin R, Accortt NA, Sandschafer D, Lawrence T, Mahtani RL. Characteristics of breast cancer patients treated with trastuzumab-anns, a trastuzumab biosimilar, in a real-world setting. Poster presented at: NCCN Virtual Annual Conference 2021; March 18-22, 2021. Accessed March 18, 2021. https://www.nccn.org/professionals/meetings/annual_conference_2021.aspx
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
