- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Study: Zarxio More Costly Than Neupogen in Outpatient Hospital Setting
According to a multiyear, retrospective study, filgrastim biosimilar Zarxio cost $20.50 more on average per administration than reference Neupogen in the hospital outpatient setting.
Costs of administering reference (Neupogen) or biosimilar filgrastim (Zarxio) are predictably higher in the outpatient hospital setting than in the office setting, although savings were nonexistent when administering the biosimilar vs reference product in the outpatient hospital setting, investigators reported.
Filgrastim is a granulocyte colony-stimulating factor drug used to prevent or treat chemotherapy induced neutropenia (lower-than-normal levels of white blood cells).
Investigators conducted a retrospective study of 65,372 episodes of filgrastim use from 2014 to 2019 among commercially covered women and men in health care settings across the United States. They sought to evaluate potential savings for use of Zarxio, tbo-filgrastim (Granix), and the reference product. Granix is approved in the European Union as a biosimilar, but in the United States it is considered a version of filgrastim because its approval did not come through the biosimilar pathway.
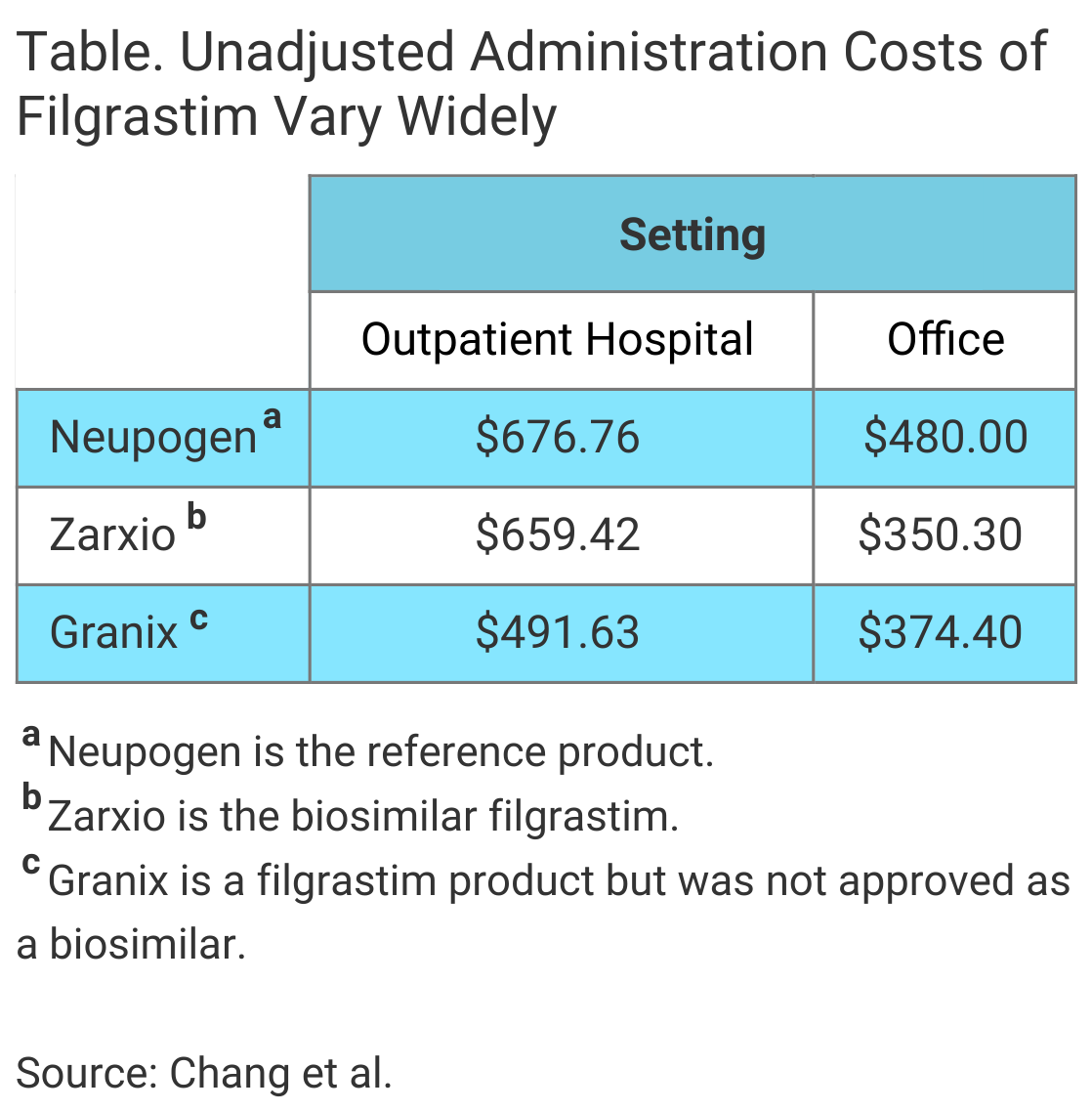
Median costs were higher in the outpatient hospital setting compared with the office setting for all 3 filgrastim products, the authors wrote in The American Journal of Managed Care® (Table). Additionally, after results were adjusted for patient characteristics and geography, the investigators found cost savings associated with the biosimilar filgrastim “were limited to the office setting, with no savings in the outpatient hospital setting.”
The authors defined an episode as 1 or more filgrastim administrations and fewer than 7 days between administrations. Median costs were adjusted for patient characteristics and geographic differences. The cost of administration was defined as the “sum of out-of-pocket and health plan paid amounts for the drug per date of service.”
In the office setting, the median costs of the biosimilar filgrastim administration and tbo-filgrastim administration were $103.61 and $94.07 less than that of reference product administration, respectively, the investigators said.
There were no cost savings associated with use of the biosimilar in the outpatient hospital setting. The adjusted median cost of biosimilar administration was greater than reference product administration by $20.50 in this setting. In contrast, the cost of tbo-filgrastim administration in the outpatient hospital setting was $132.90 less than a reference product administration.
Although previous analyses have found lower costs for the biosimilar filgrastim than for reference filgrastim, the authors concluded their data suggest that “site of care can change this calculus, reducing savings.”
One possible contributing factor they suggested is the difference in payer payments for outpatient- vs office-based treatment.
"Although patients receive identical drug administrations in office or hospital outpatient settings, insurers generally reimburse higher payments to outpatient hospital settings to cover presumed greater overhead costs," they wrote.
The authors said that despite their findings being limited to commercially covered patients, the results suggest that “savings from biosimilar drugs are context specific,” although they said it is unknown whether the cost savings for other biosimilar drugs vary similarly by site of care.
“Biosimilars—drugs that are similar in quality, safety, and efficacy to reference biologic drugs—are intended to increase competition, reduce prices, and improve patient access to important treatments,” they said. They did not speculate on why in the outpatient hospital setting the cost of Zarxio administration was slightly higher, on average, than reference product administration.
They also did not speculate on why tbo-filgrastim was significantly less costly than reference filgrastim in both the outpatient and office settings, but not the biosimilar filgrastim. In addition to Neupogen, Zarxio, and Granix, another filgrastim product is available, the biosimilar Nivestym. This was approved and launched in late 2018, which was midway through the study period. Investigators did not review cost data for Nivestym.
Granix was approved in August 2012 and launched in November 2013, and Zarxio in March 2015 and September 2015, respectively.
Reference
Chang J, Karaca-Mandic P, Go RS, Schondelmeyer S, Weisdorf D, Jeffery MM. Site of care potentially limits cost savings from biosimilars. Am J Manag Care. 2021;27(8):e287-e289. doi:10.37765/ajmc.2021.88730
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.